Deposition Date
2006-01-16
Release Date
2006-05-23
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2FPB
Keywords:
Title:
Structure of Strictosidine Synthase, the Biosynthetic Entry to the Monoterpenoid Indole Alkaloid Family
Biological Source:
Source Organism(s):
Rauvolfia serpentina (Taxon ID: 4060)
Expression System(s):
Method Details:
Experimental Method:
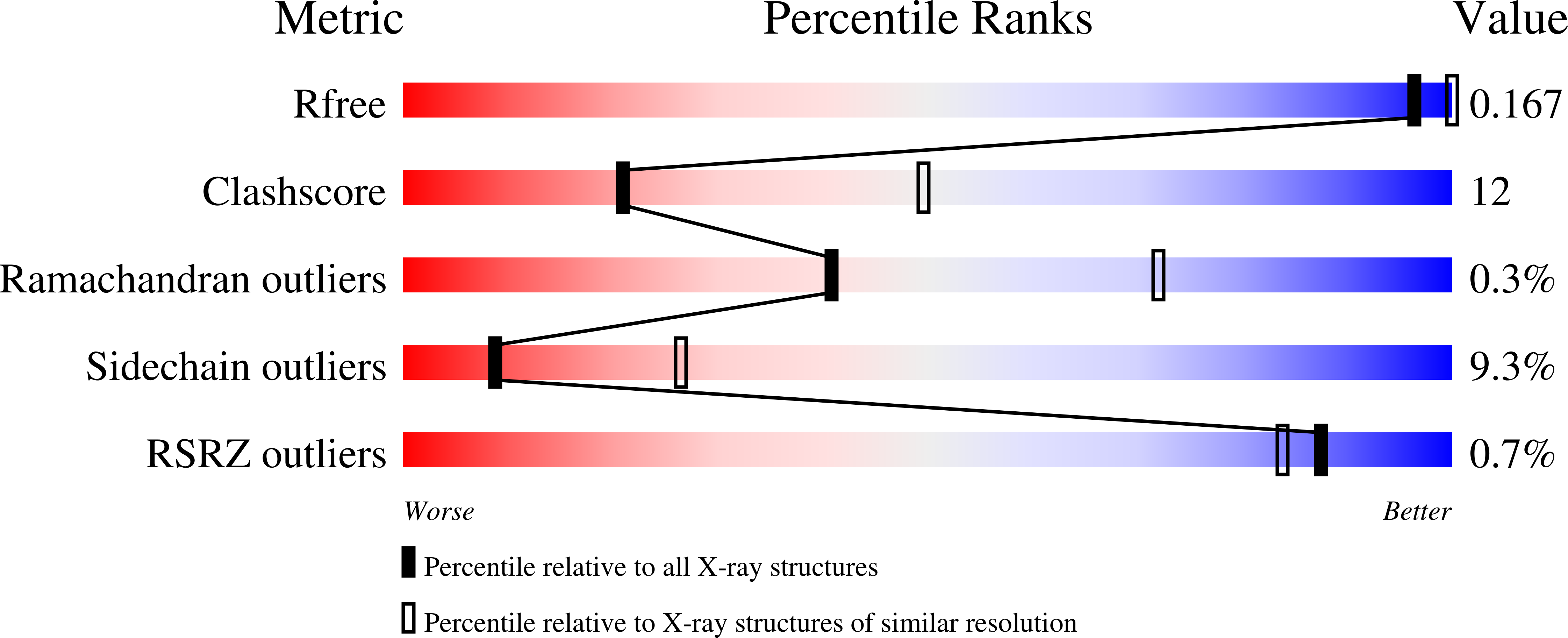
Resolution:
2.80 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
H 3