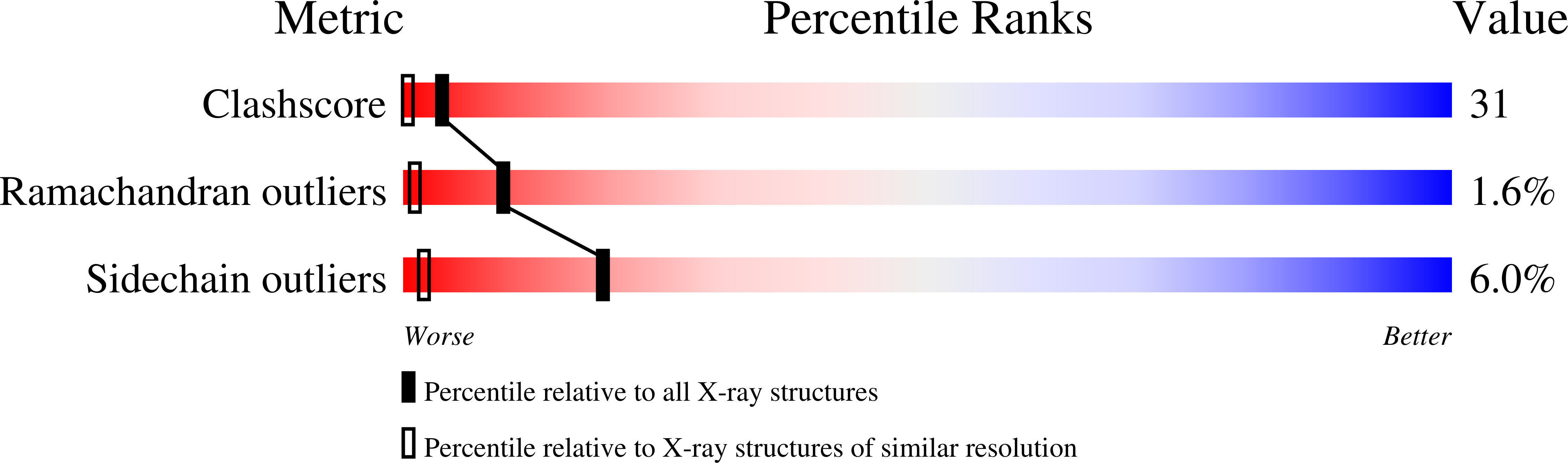Deposition Date
2006-01-11
Release Date
2006-10-03
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2FNW
Keywords:
Title:
Pseudomonas aeruginosa E2Q/H83Q/M109H-azurin RE(PHEN)(CO)3
Biological Source:
Source Organism:
Pseudomonas aeruginosa (Taxon ID: 287)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.40 Å
R-Value Free:
0.22
R-Value Work:
0.19
Space Group:
P 1