Deposition Date
2005-12-19
Release Date
2006-06-20
Last Version Date
2023-08-30
Entry Detail
PDB ID:
2FFF
Keywords:
Title:
Open Form of a Class A Transpeptidase Domain
Biological Source:
Source Organism(s):
Streptococcus pneumoniae (Taxon ID: 1313)
Expression System(s):
Method Details:
Experimental Method:
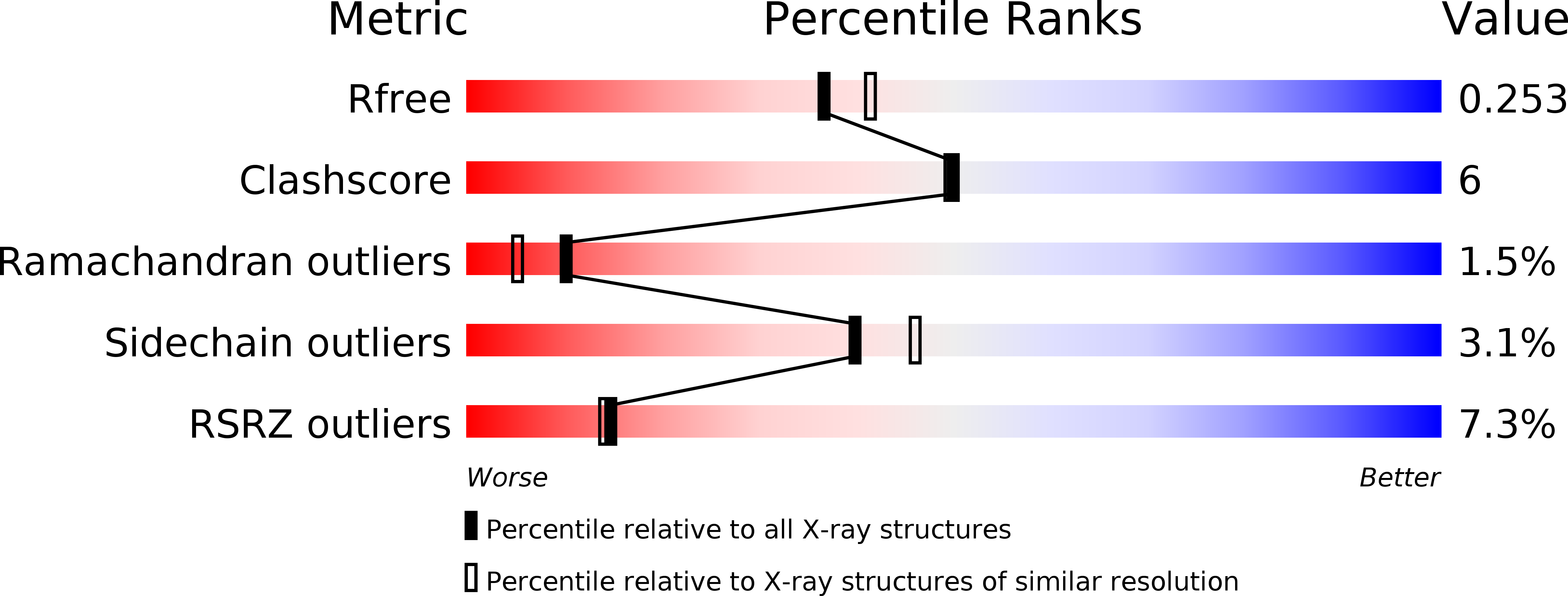
Resolution:
2.23 Å
R-Value Free:
0.24
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1