Deposition Date
2005-12-12
Release Date
2006-01-31
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2FCN
Keywords:
Title:
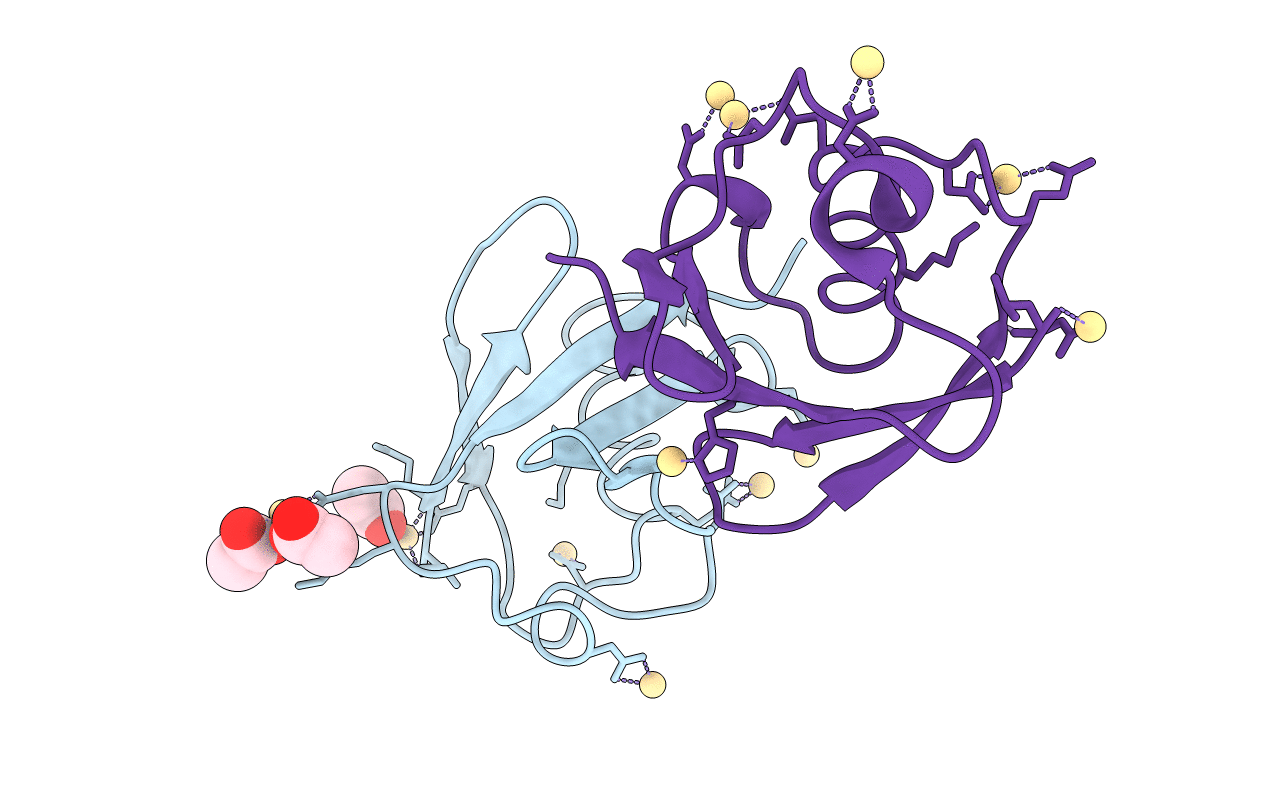
X-ray Crystal Structure of a Chemically Synthesized [D-Val35]Ubiquitin with a Cubic Space Group
Method Details:
Experimental Method:
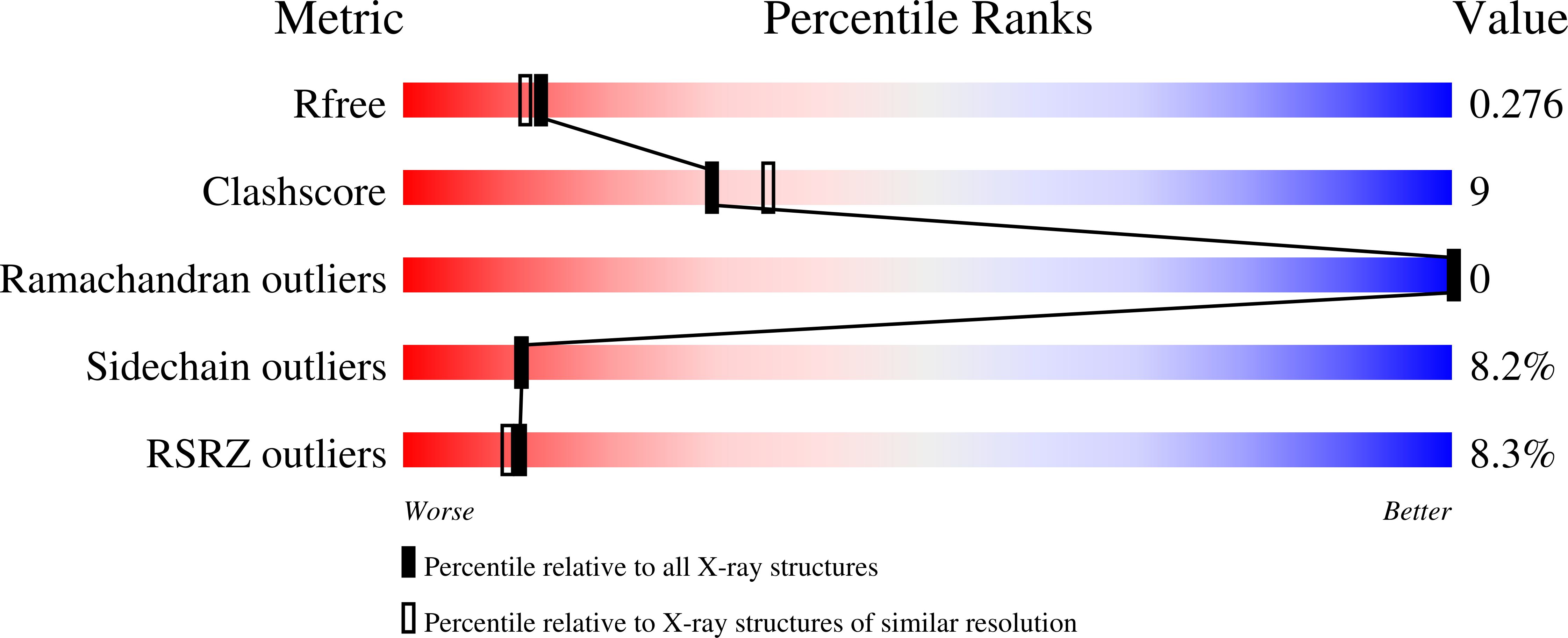
Resolution:
2.20 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 43 3 2