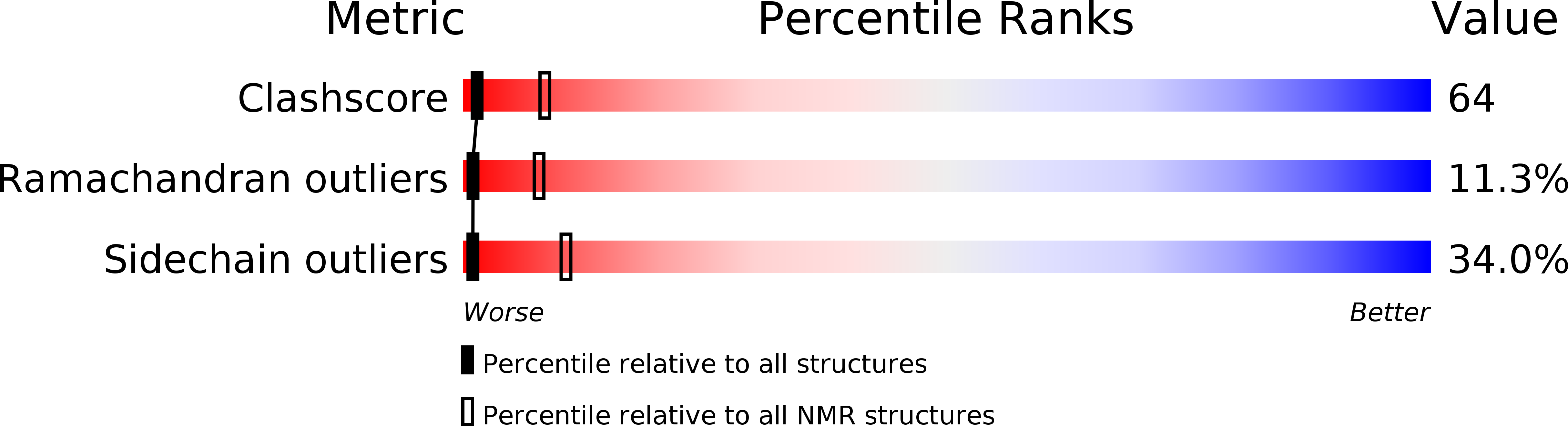
Deposition Date
2005-12-12
Release Date
2006-01-31
Last Version Date
2023-11-15
Entry Detail
PDB ID:
2FCI
Keywords:
Title:
Structural basis for the requirement of two phosphotyrosines in signaling mediated by Syk tyrosine kinase
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
16
Selection Criteria:
Lack of NOE violations & correct main chain geometry