Deposition Date
2005-11-23
Release Date
2006-04-11
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2F4K
Keywords:
Title:
Chicken villin subdomain HP-35, K65(NLE), N68H, K70(NLE), PH9
Method Details:
Experimental Method:
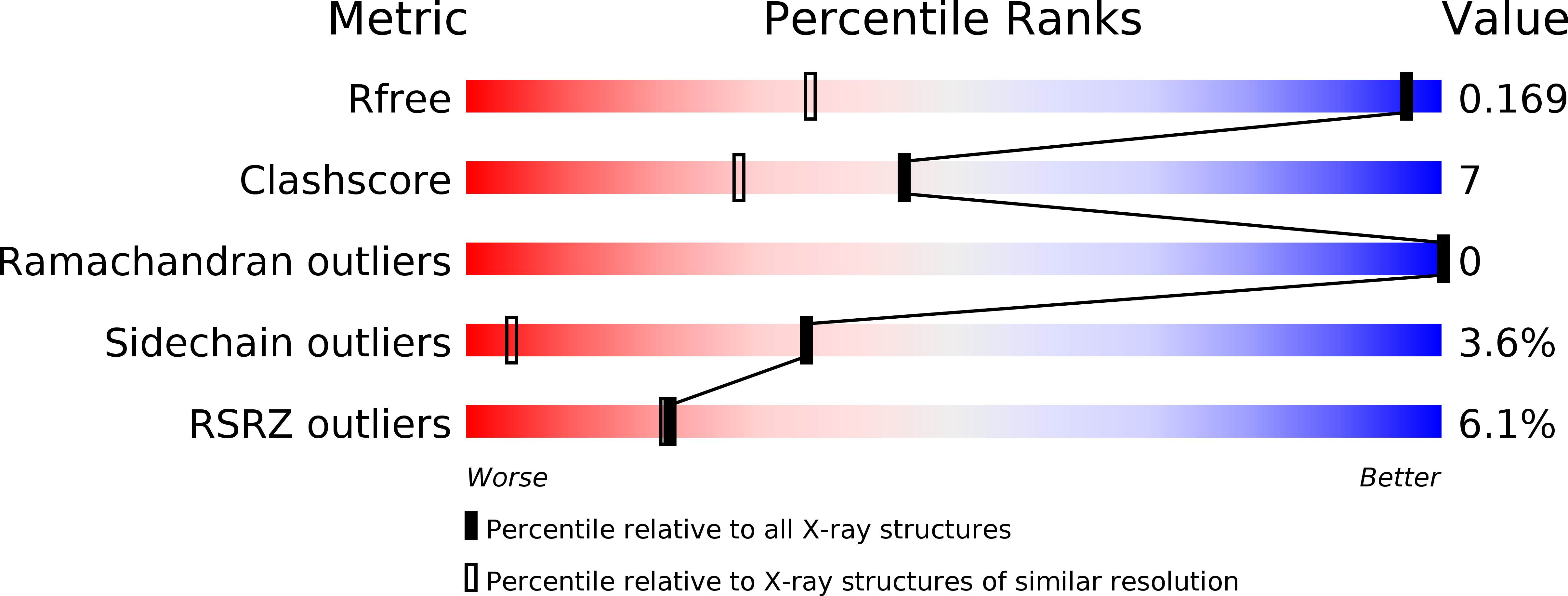
Resolution:
1.05 Å
R-Value Free:
0.16
R-Value Observed:
0.14
Space Group:
C 2 2 21