Deposition Date
2005-11-14
Release Date
2006-12-05
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2F1A
Keywords:
Title:
GOLGI ALPHA-MANNOSIDASE II COMPLEX WITH (2R,3R,4S)-2-({[(1S)-2-hydroxy-1-phenylethyl]amino}methyl)pyrrolidine-3,4-diol
Biological Source:
Source Organism(s):
Drosophila melanogaster (Taxon ID: 7227)
Expression System(s):
Method Details:
Experimental Method:
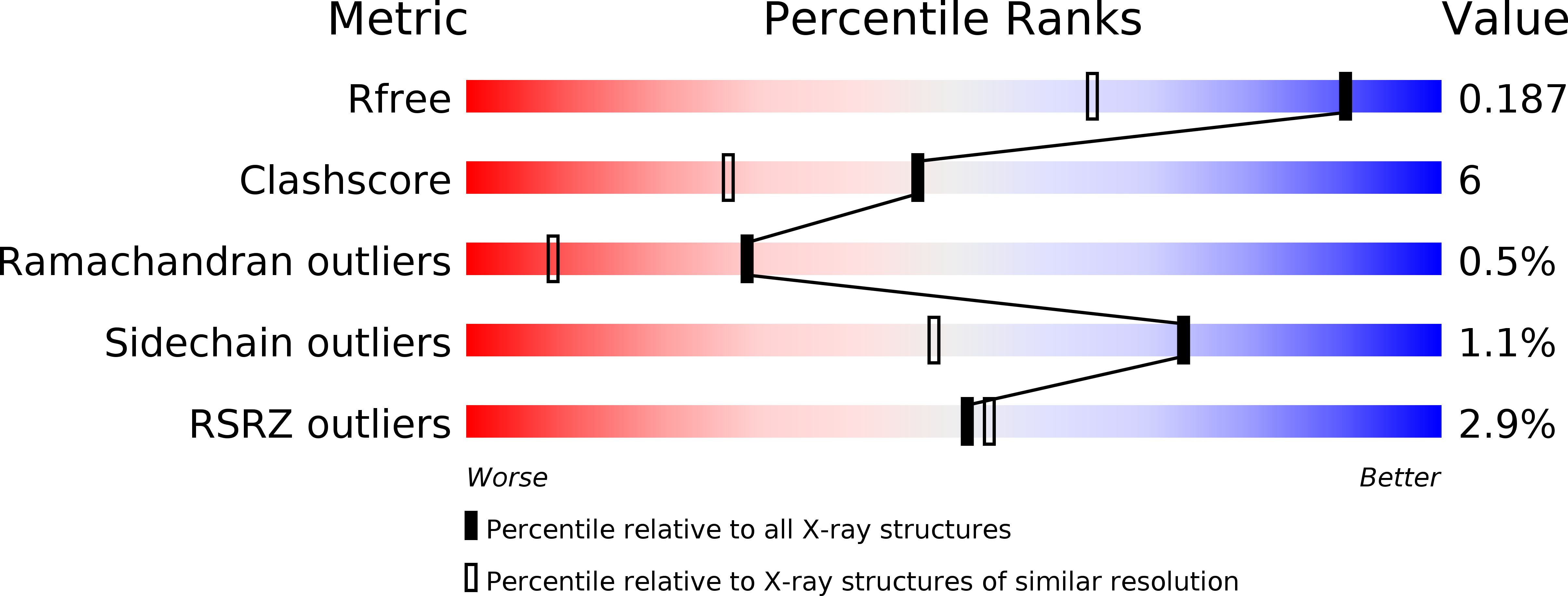
Resolution:
1.45 Å
R-Value Free:
0.18
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21