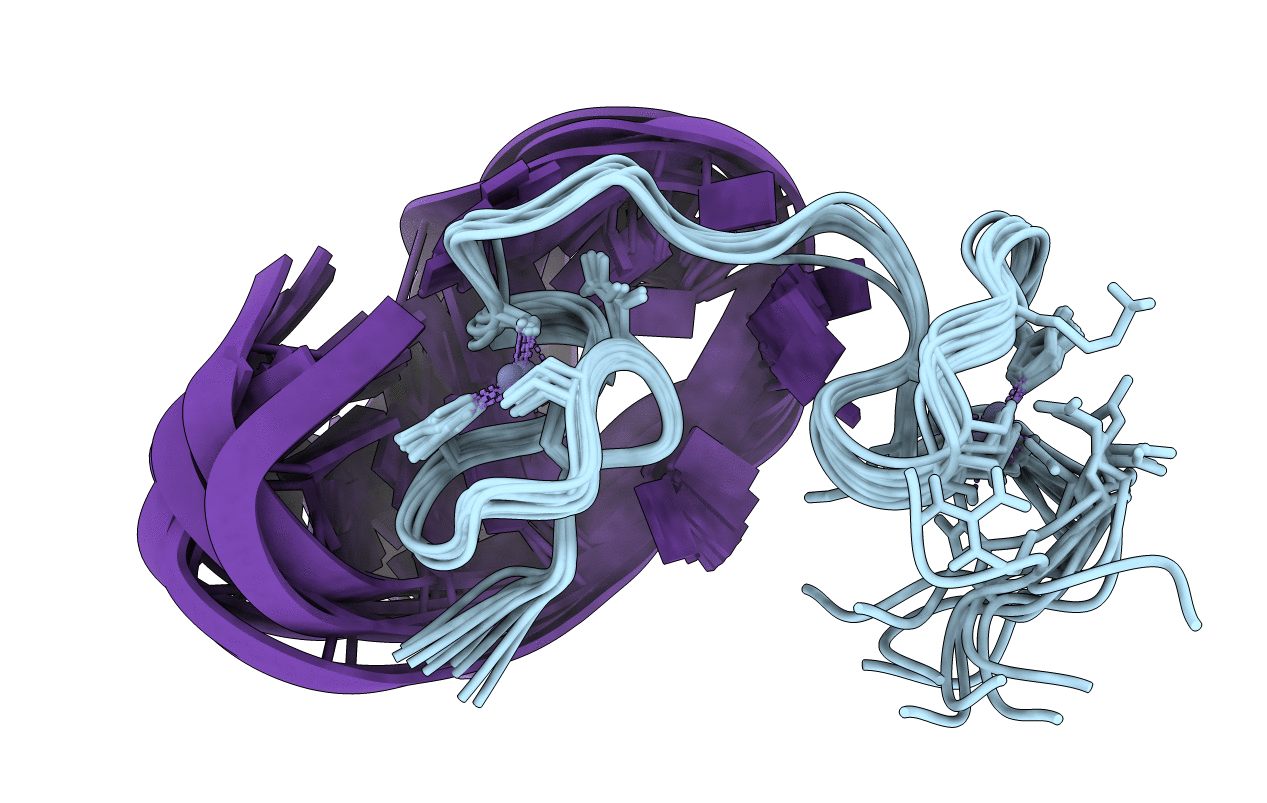
Deposition Date
2005-11-08
Release Date
2007-04-24
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2EXF
Keywords:
Title:
Solution structure of the HIV-1 nucleocapsid (NCp7(12-55)) complexed with the DNA (-) Primer Binding Site
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with acceptable covalent geometry,structures with favorable non-bond energy,structures with the least restraint violations,structures with the lowest energy