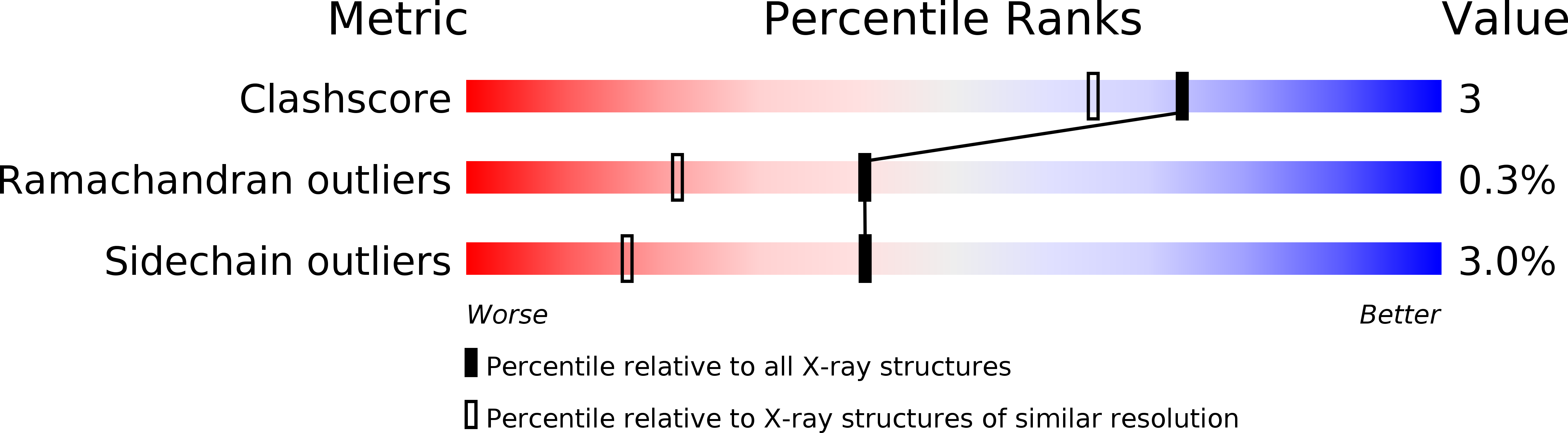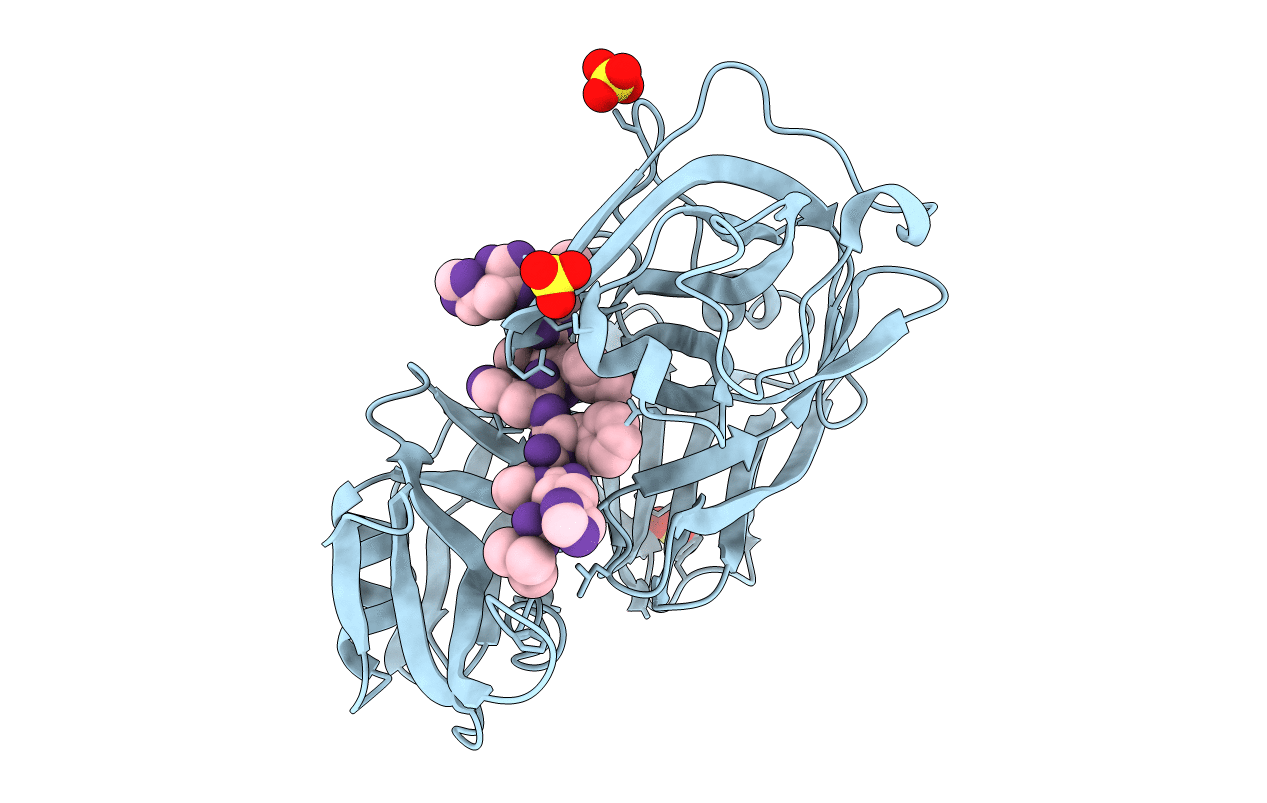
Deposition Date
1990-11-12
Release Date
1991-01-15
Last Version Date
2023-11-15
Entry Detail
PDB ID:
2ER7
Keywords:
Title:
X-RAY ANALYSES OF ASPARTIC PROTEINASES.III. THREE-DIMENSIONAL STRUCTURE OF ENDOTHIAPEPSIN COMPLEXED WITH A TRANSITION-STATE ISOSTERE INHIBITOR OF RENIN AT 1.6 ANGSTROMS RESOLUTION
Biological Source:
Source Organism(s):
Cryphonectria parasitica (Taxon ID: 5116)
Method Details: