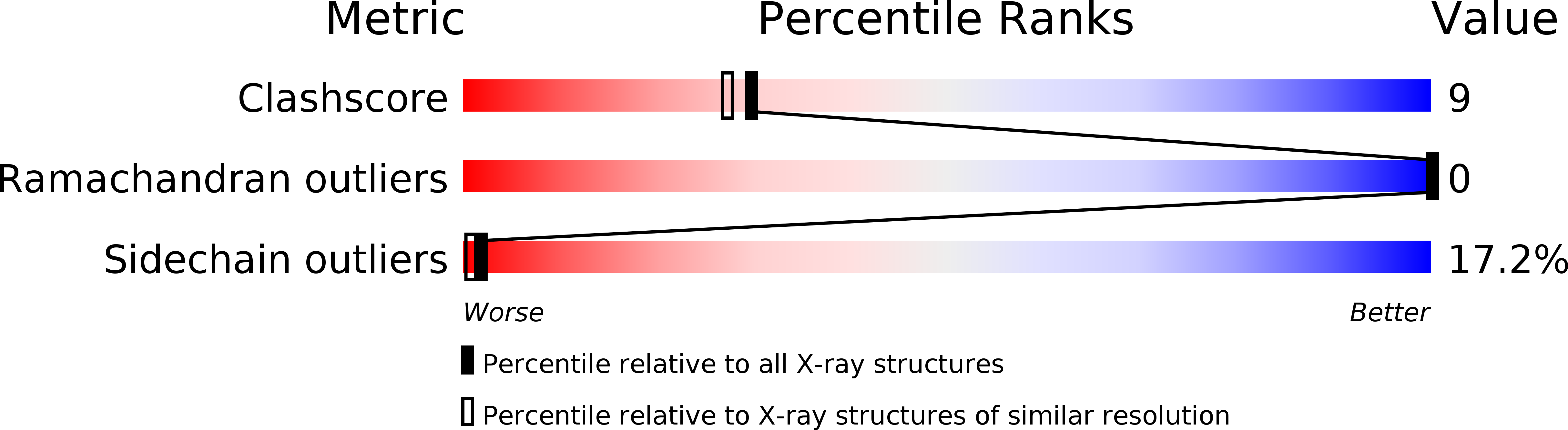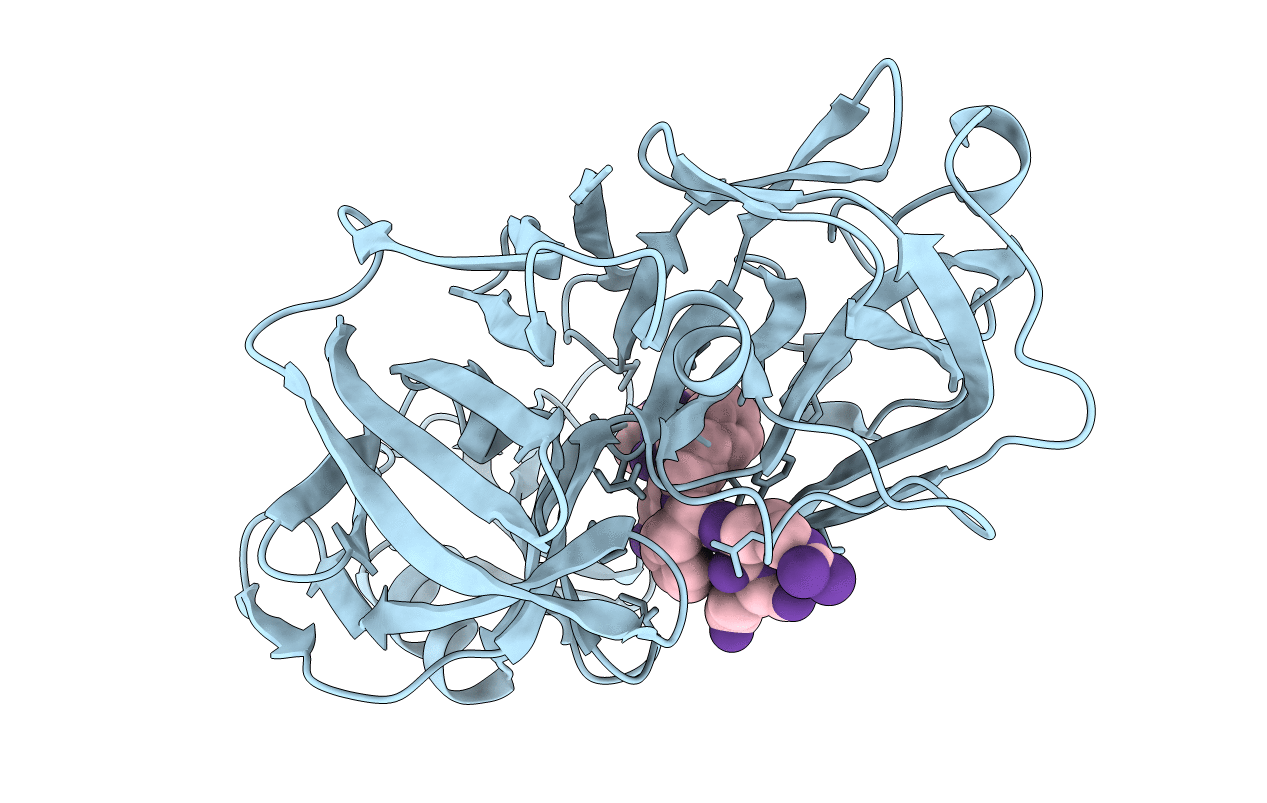
Deposition Date
1990-10-13
Release Date
1991-01-15
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2ER6
Keywords:
Title:
The structure of a synthetic pepsin inhibitor complexed with endothiapepsin.
Biological Source:
Source Organism(s):
Cryphonectria parasitica (Taxon ID: 5116)
Method Details: