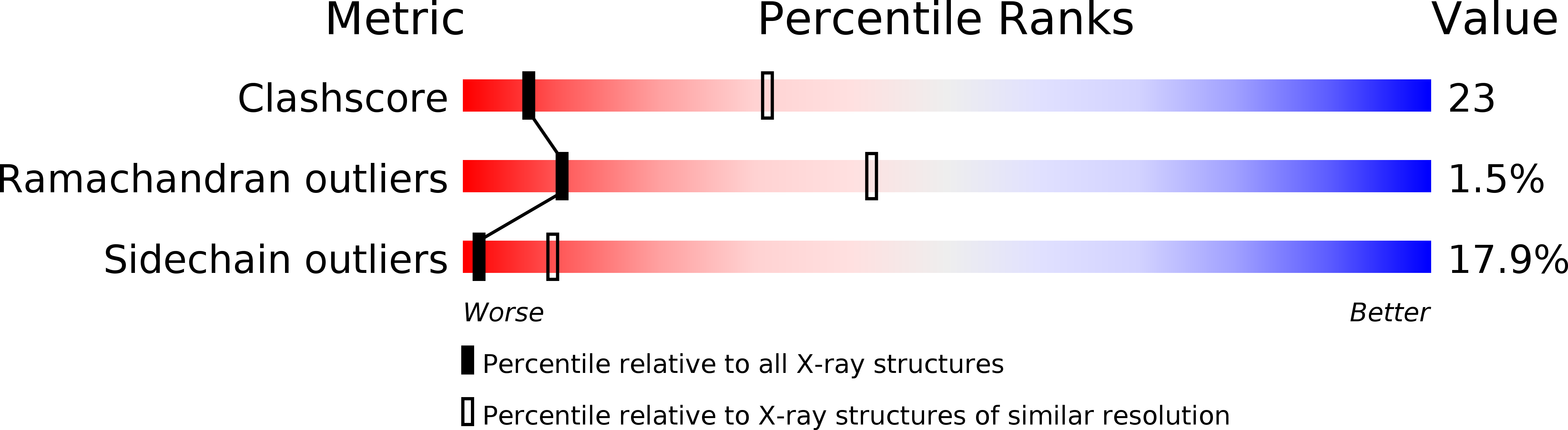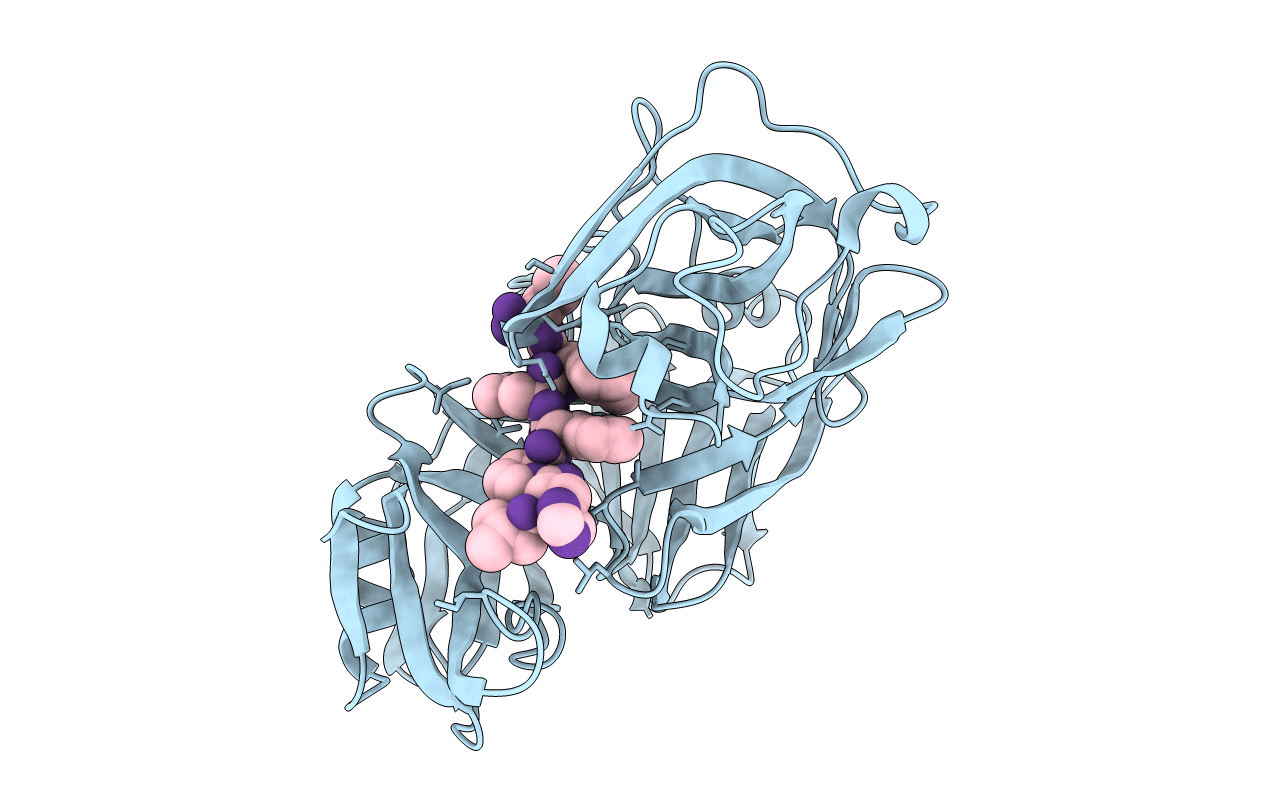
Deposition Date
1990-10-20
Release Date
1991-01-15
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2ER0
Keywords:
Title:
X-RAY STUDIES OF ASPARTIC PROTEINASE-STATINE INHIBITOR COMPLEXES
Biological Source:
Source Organism(s):
Cryphonectria parasitica (Taxon ID: 5116)
Method Details: