Deposition Date
2007-02-09
Release Date
2007-06-26
Last Version Date
2024-11-13
Entry Detail
PDB ID:
2EBS
Keywords:
Title:
Crystal Structure Anaalysis of Oligoxyloglucan reducing-end-specific cellobiohydrolase (OXG-RCBH) D465N Mutant Complexed with a Xyloglucan Heptasaccharide
Biological Source:
Source Organism(s):
Geotrichum sp. M128 (Taxon ID: 203496)
Expression System(s):
Method Details:
Experimental Method:
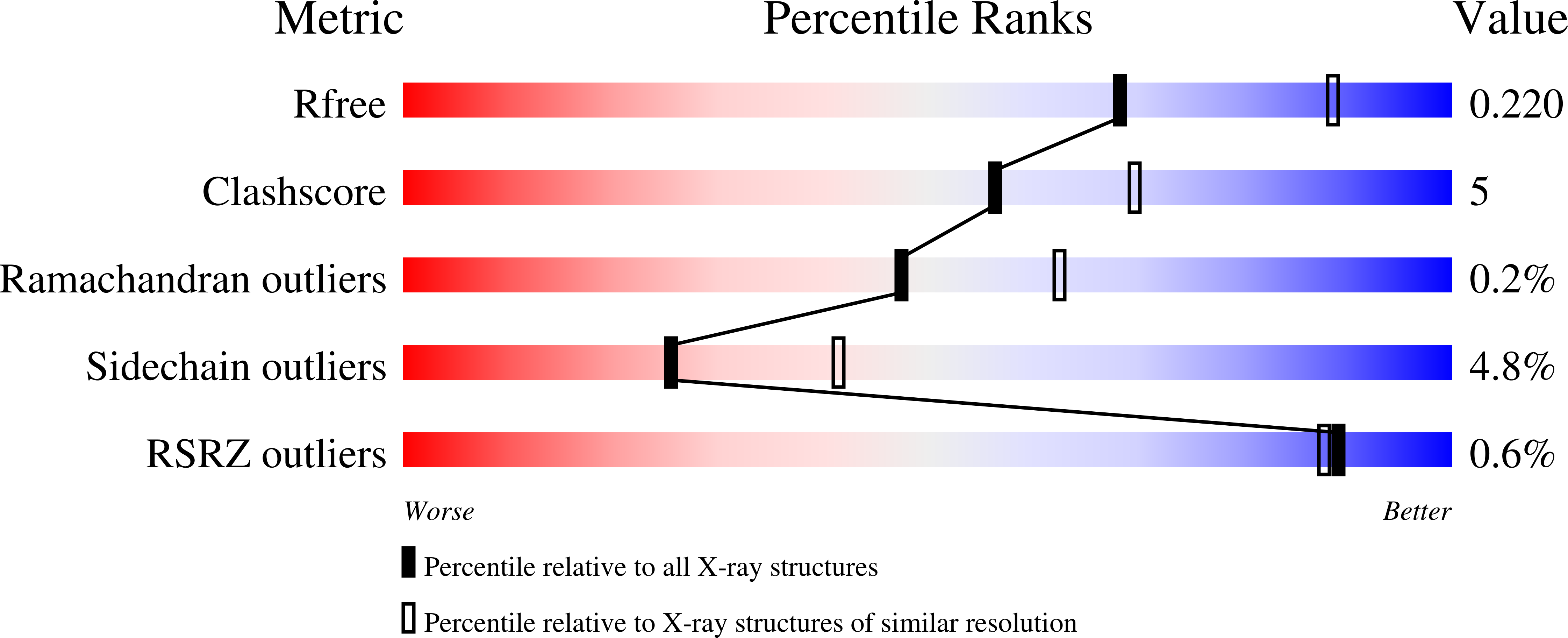
Resolution:
2.40 Å
R-Value Free:
0.21
R-Value Work:
0.15
R-Value Observed:
0.16
Space Group:
P 21 21 21