Deposition Date
2006-05-13
Release Date
2007-04-24
Last Version Date
2024-04-03
Entry Detail
PDB ID:
2DPR
Keywords:
Title:
The crystal structures of the calcium-bound con-G and con-T(K7Glu) dimeric peptides demonstrate a novel metal-dependent helix-forming motif
Biological Source:
Source Organism(s):
Conus tulipa (Taxon ID: 6495)
Method Details:
Experimental Method:
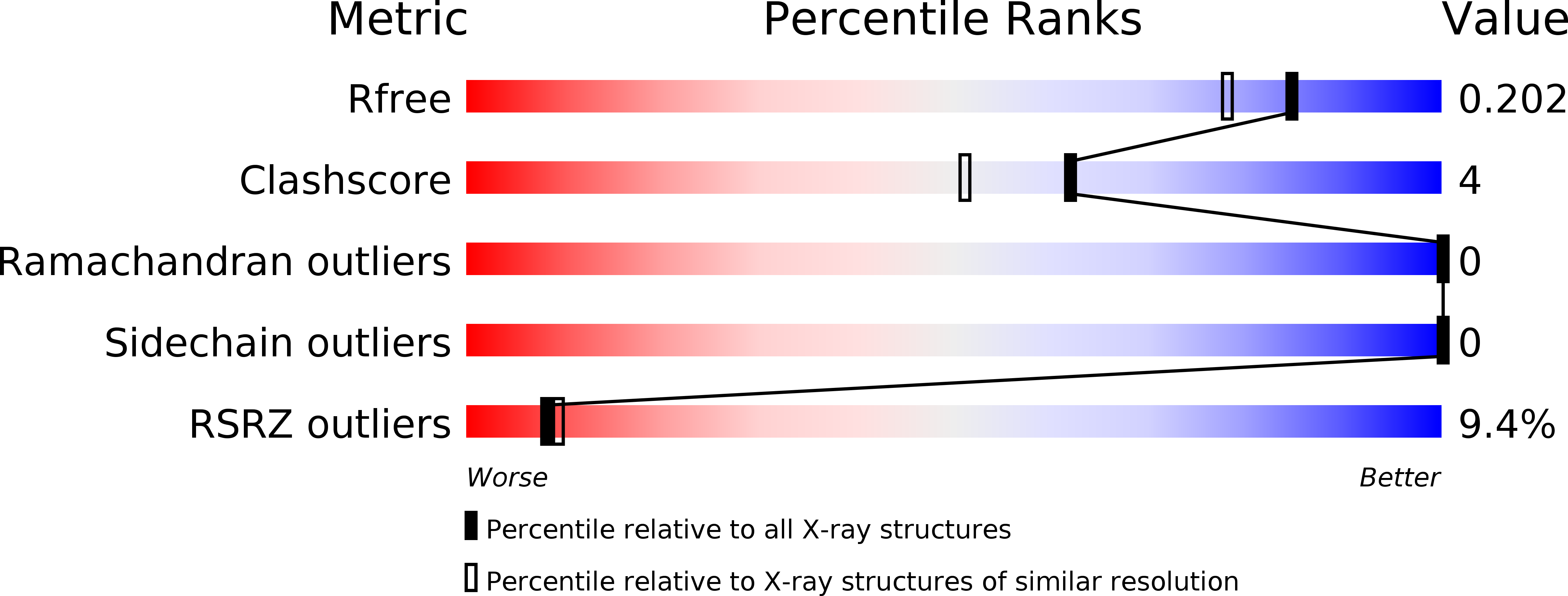
Resolution:
1.70 Å
R-Value Free:
0.18
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 43 3 2