Deposition Date
2005-09-30
Release Date
2006-09-30
Last Version Date
2024-03-13
Entry Detail
PDB ID:
2D3Q
Keywords:
Title:
Crystal Structure of a Decolorizing Peroxidase (DyP) That Catalyses the Biological Oxidation of Anthraquinone Derivatives
Biological Source:
Source Organism(s):
Bjerkandera adusta (Taxon ID: 5331)
Expression System(s):
Method Details:
Experimental Method:
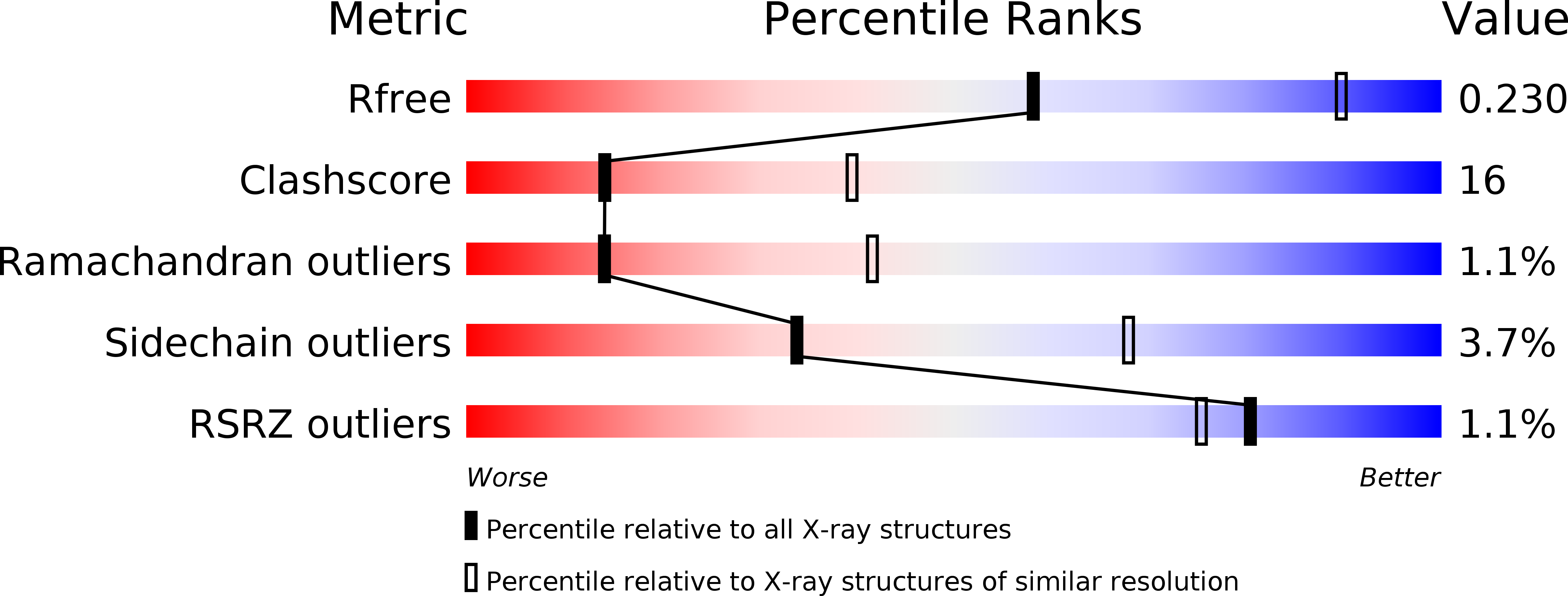
Resolution:
2.80 Å
R-Value Free:
0.26
R-Value Work:
0.23
R-Value Observed:
0.26
Space Group:
P 65 2 2