Deposition Date
2006-05-25
Release Date
2006-06-27
Last Version Date
2024-05-01
Entry Detail
PDB ID:
2CO2
Keywords:
Title:
Salmonella enterica SafA pilin in complex with a 19-residue SafA Nte peptide (F3A mutant)
Biological Source:
Source Organism(s):
SALMONELLA ENTERICA (Taxon ID: 28901)
Expression System(s):
Method Details:
Experimental Method:
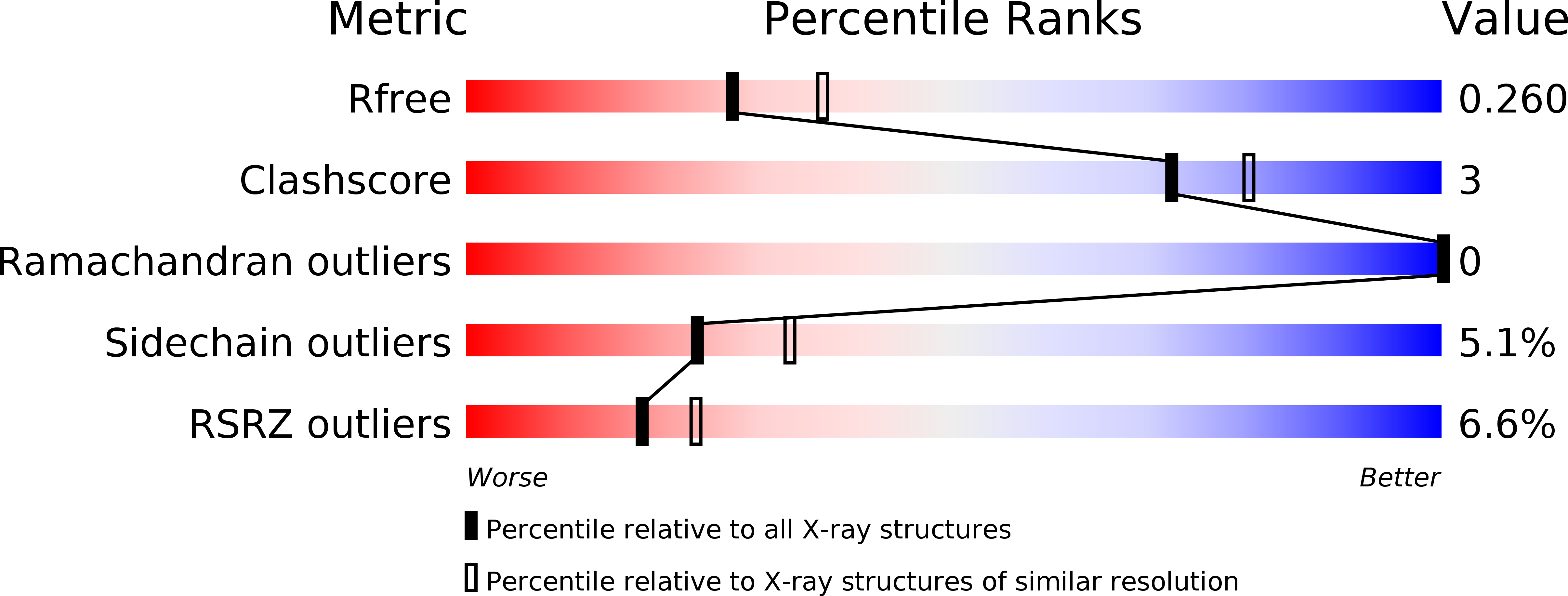
Resolution:
2.30 Å
R-Value Free:
0.26
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 61 2 2