Deposition Date
2006-03-24
Release Date
2006-07-12
Last Version Date
2024-11-13
Entry Detail
PDB ID:
2CIS
Keywords:
Title:
Structure-based functional annotation: Yeast ymr099c codes for a D- hexose-6-phosphate mutarotase. Complex with tagatose-6-phosphate
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 559292)
Expression System(s):
Method Details:
Experimental Method:
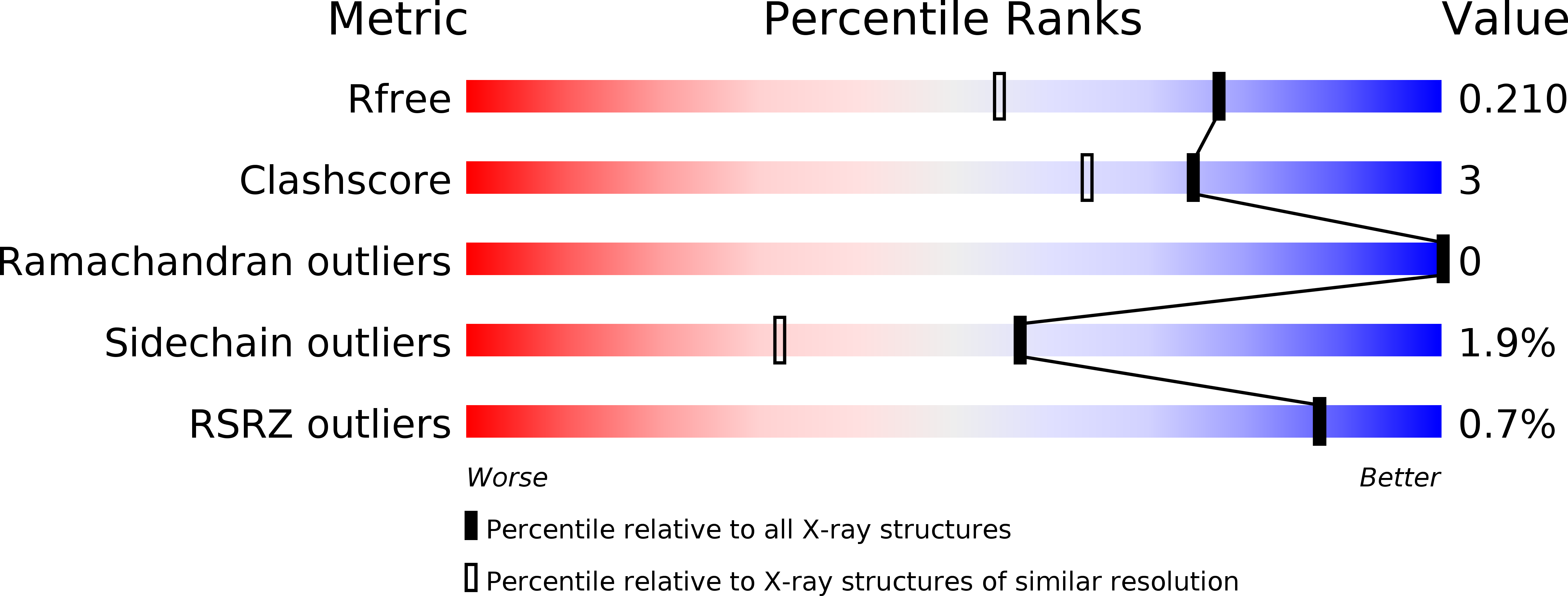
Resolution:
1.62 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 2 2 21