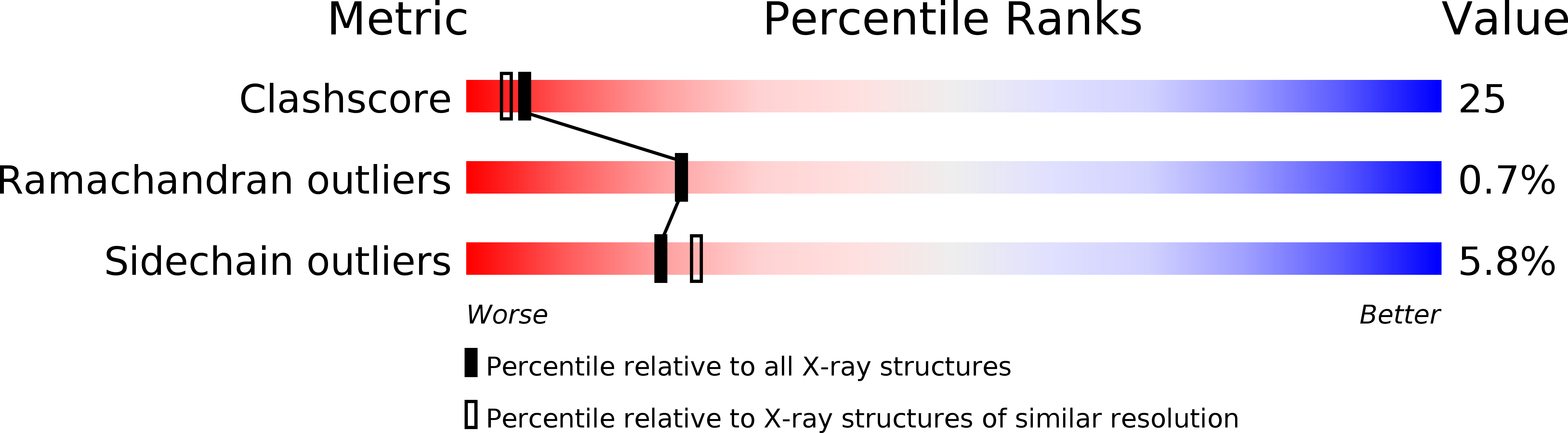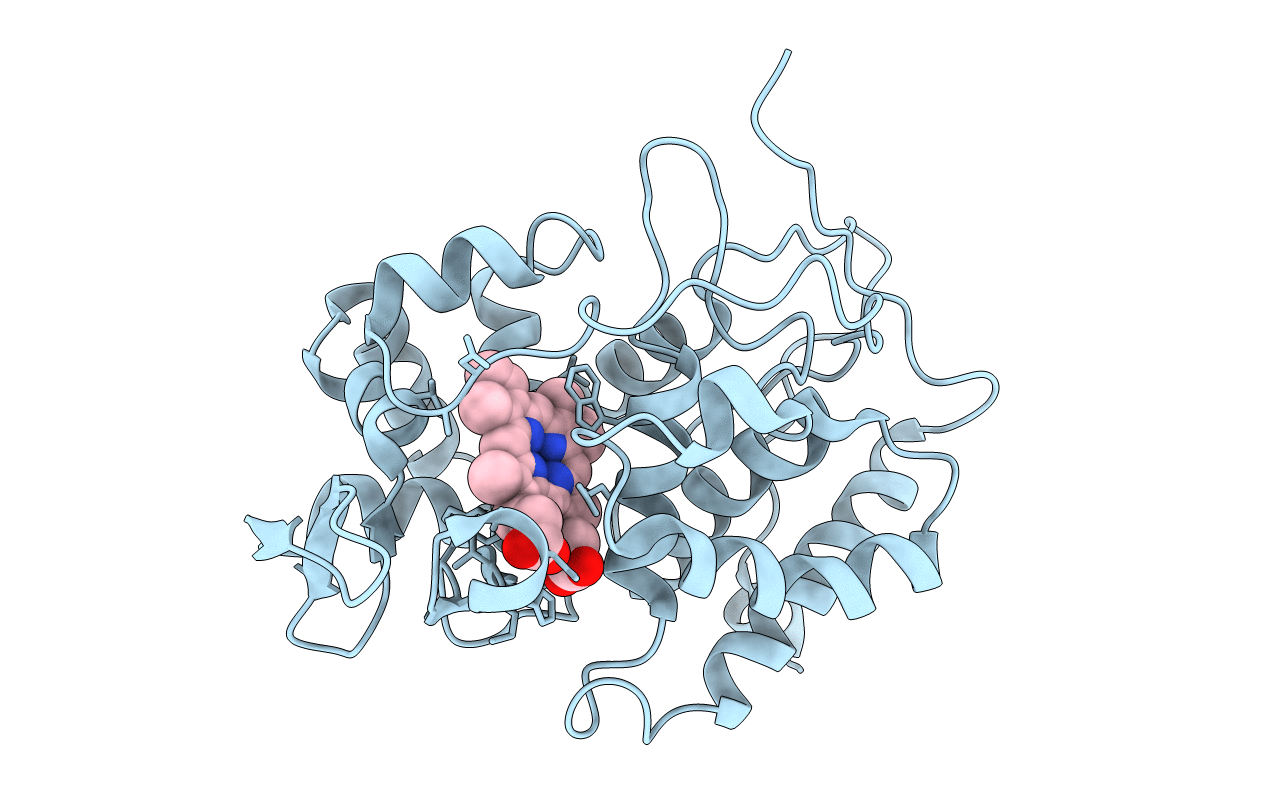
Deposition Date
1994-05-31
Release Date
1994-08-31
Last Version Date
2024-02-14
Entry Detail
PDB ID:
2CEP
Keywords:
Title:
ROLE OF MET-230 IN INTRAMOLECULAR ELECTRON TRANSFER BETWEEN THE OXYFERRYL HEME AND TRP 191 IN CYTOCHROME C PEROXIDASE COMPOUND II
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Method Details:
Experimental Method:
Resolution:
2.20 Å
R-Value Observed:
0.16
Space Group:
P 21 21 21