Deposition Date
2006-01-18
Release Date
2006-05-15
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2CCV
Keywords:
Title:
Structure of Helix Pomatia agglutinin with zinc and N-acetyl-alpha-D- galactoseamine (GalNAc)
Biological Source:
Source Organism(s):
HELIX POMATIA (Taxon ID: 6536)
Method Details:
Experimental Method:
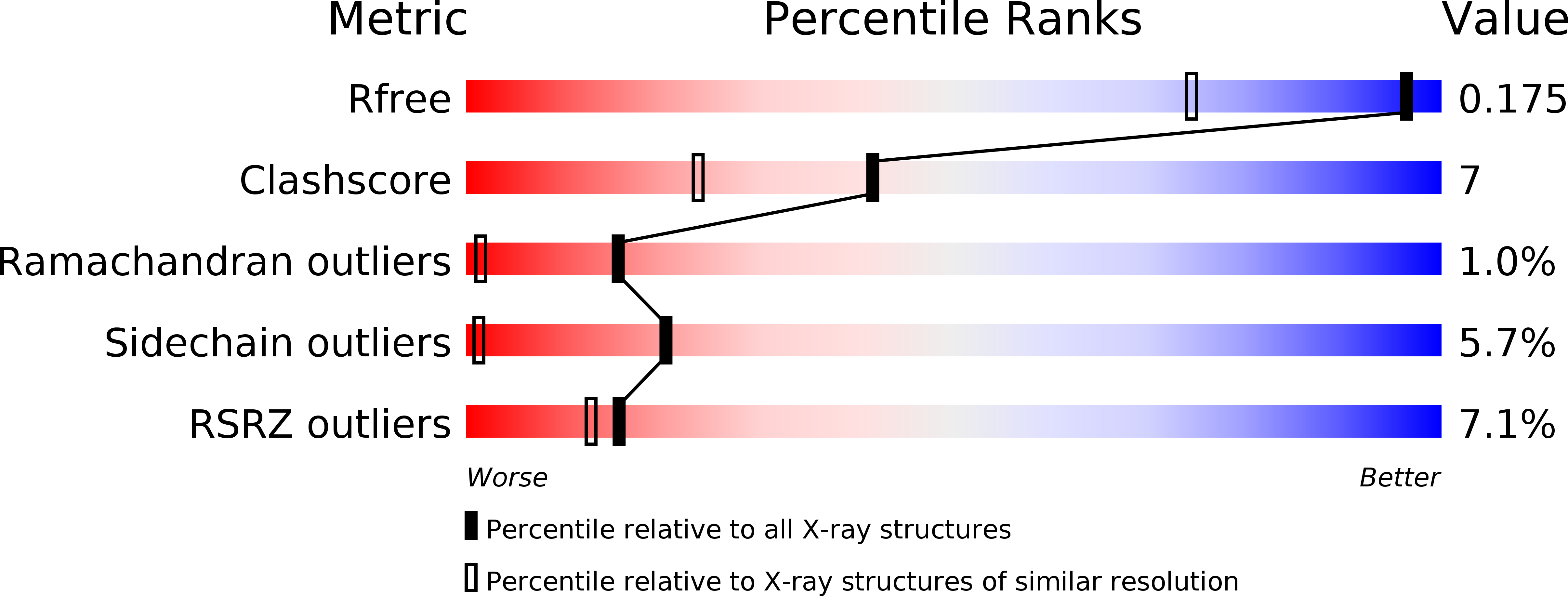
Resolution:
1.30 Å
R-Value Free:
0.17
R-Value Observed:
0.14
Space Group:
H 3 2