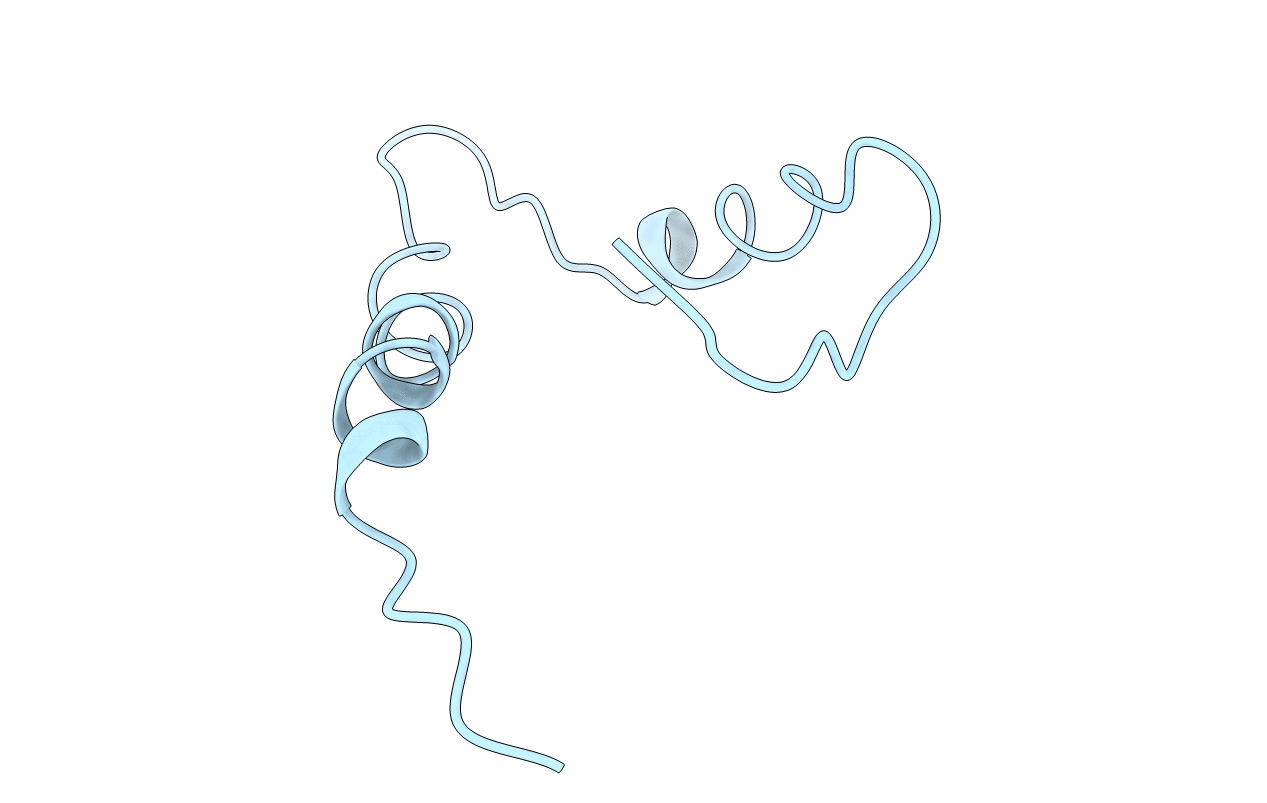
Deposition Date
2005-10-25
Release Date
2005-11-02
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2C55
Keywords:
Title:
Solution Structure of the Human Immunodeficiency Virus Type 1 p6 Protein
Biological Source:
Source Organism(s):
HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 (Taxon ID: 11676)
Method Details:
Experimental Method:
Conformers Calculated:
104
Conformers Submitted:
1
Selection Criteria:
LEAST RESTRAINT VIOLATION


