Deposition Date
2005-09-23
Release Date
2006-02-01
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2C21
Keywords:
Title:
Specificity of the Trypanothione-dependednt Leishmania major Glyoxalase I: Structure and biochemical comparison with the human enzyme
Biological Source:
Source Organism(s):
LEISHMANIA MAJOR (Taxon ID: 5664)
Expression System(s):
Method Details:
Experimental Method:
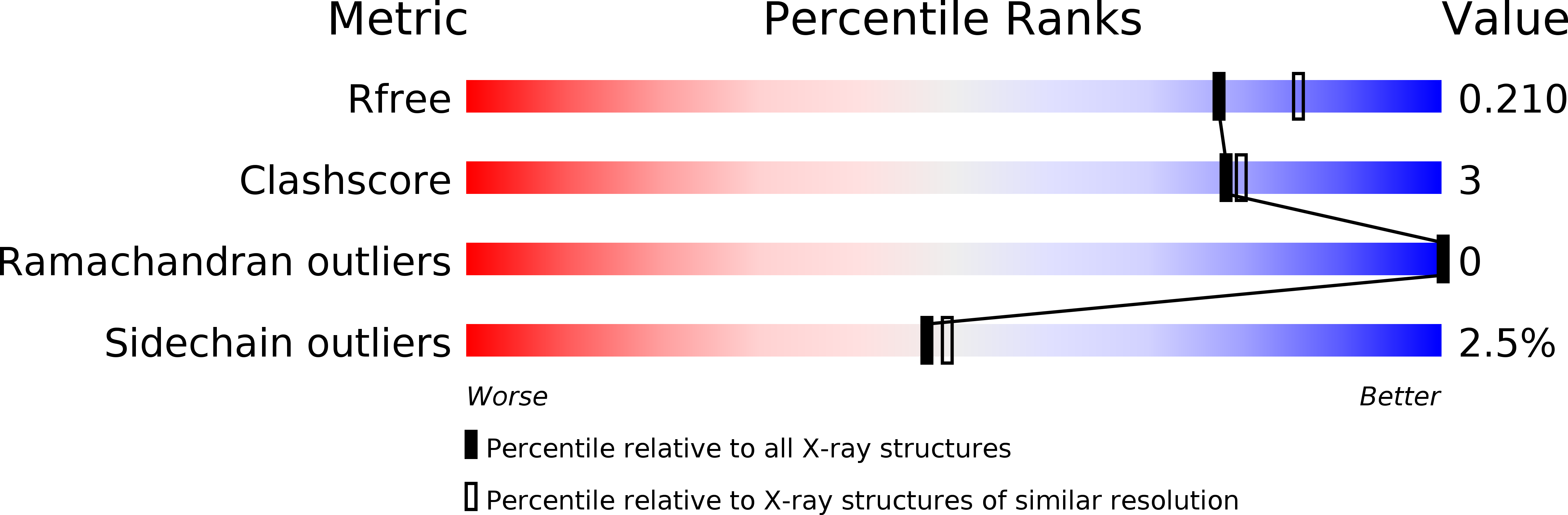
Resolution:
2.00 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 21 21 2