Deposition Date
2005-05-18
Release Date
2005-08-19
Last Version Date
2024-11-13
Entry Detail
PDB ID:
2BS8
Keywords:
Title:
Crystal structure of F17b-G in complex with N-acetyl-D-glucosamine
Biological Source:
Source Organism(s):
ESCHERICHIA COLI B (Taxon ID: 37762)
Expression System(s):
Method Details:
Experimental Method:
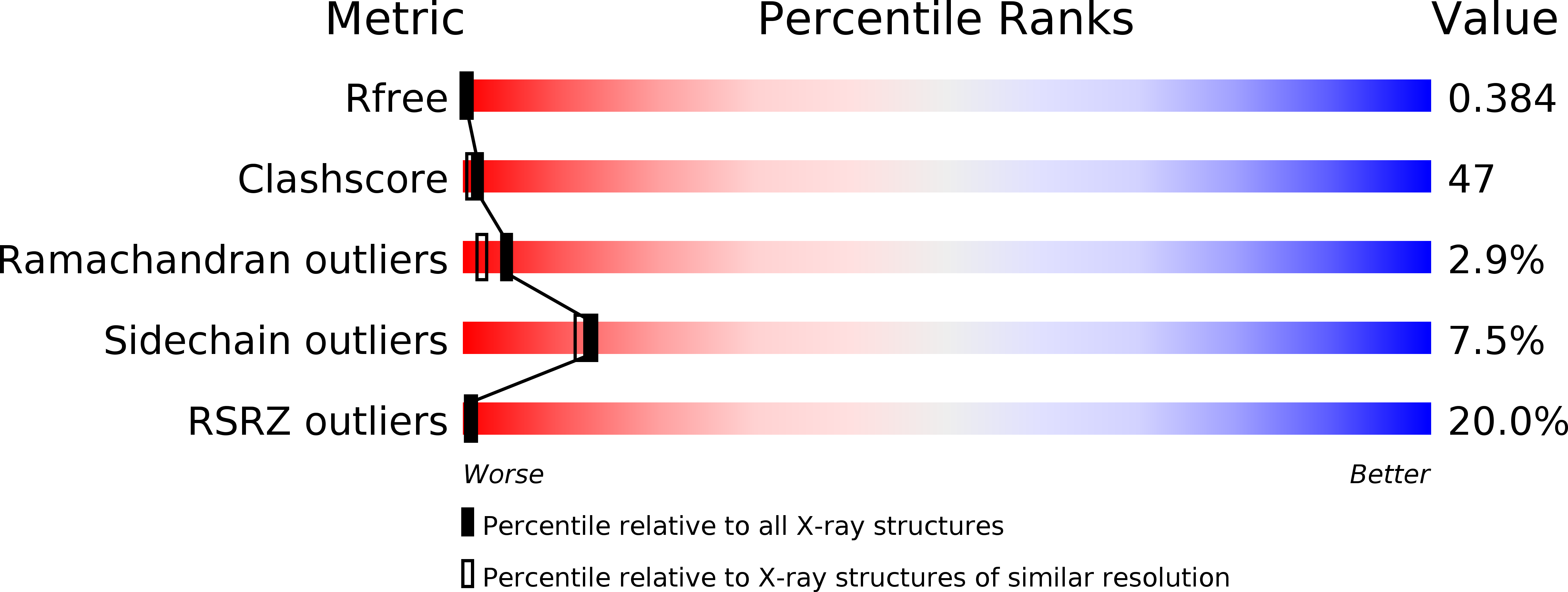
Resolution:
2.25 Å
R-Value Free:
0.39
R-Value Work:
0.33
R-Value Observed:
0.33
Space Group:
I 41 2 2