Deposition Date
2005-05-03
Release Date
2005-06-07
Last Version Date
2024-11-06
Entry Detail
PDB ID:
2BR8
Keywords:
Title:
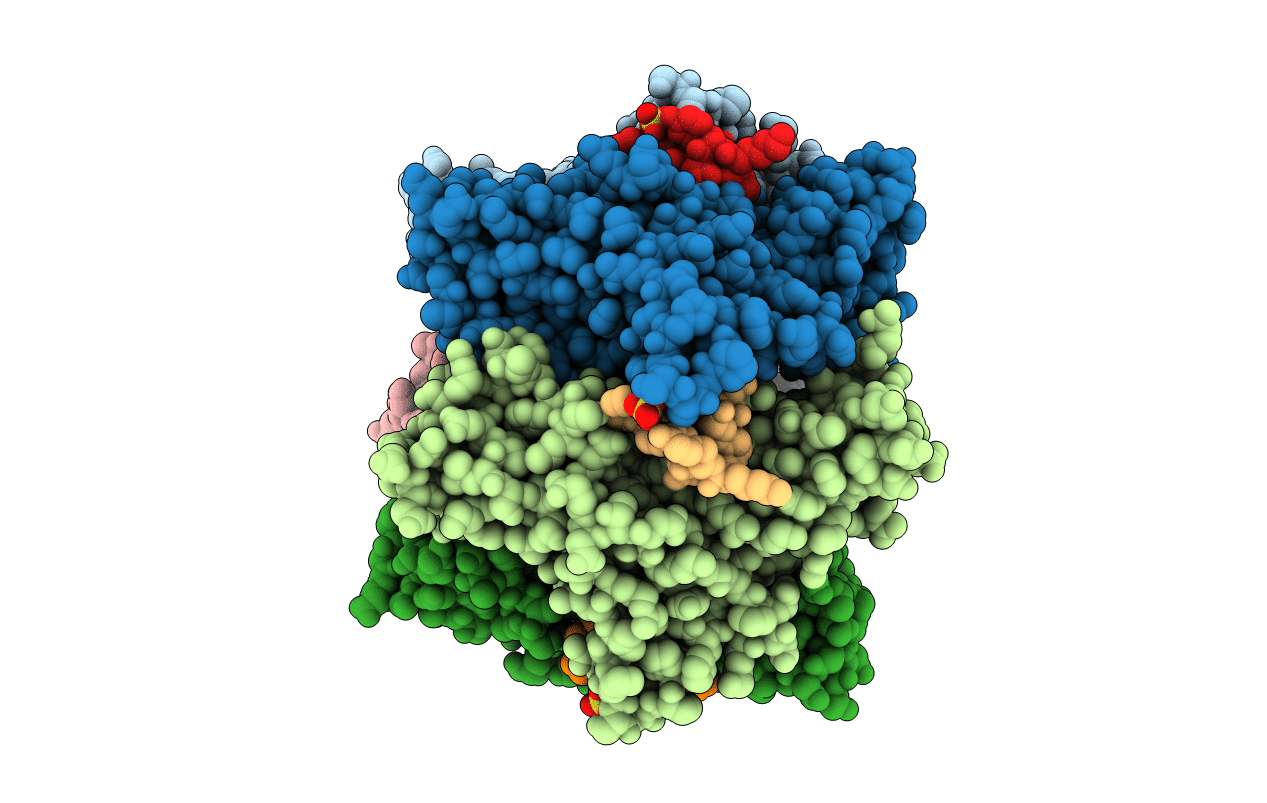
Crystal Structure of Acetylcholine-binding Protein (AChBP) from Aplysia californica in complex with an alpha-conotoxin PnIA variant
Biological Source:
Source Organism(s):
APLYSIA CALIFORNICA (Taxon ID: 6500)
CONUS PENNACEUS (Taxon ID: 37335)
CONUS PENNACEUS (Taxon ID: 37335)
Expression System(s):
Method Details:
Experimental Method:
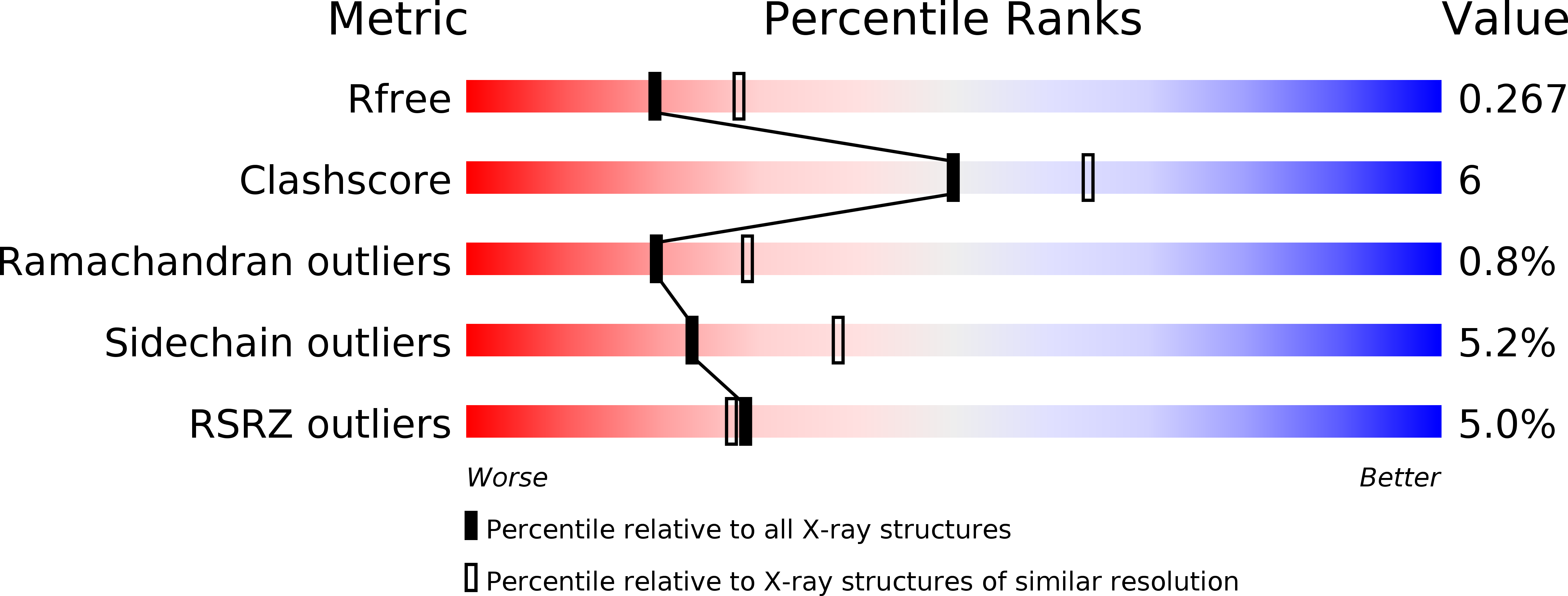
Resolution:
2.40 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 1 21 1