Deposition Date
2005-04-06
Release Date
2006-03-15
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2BNZ
Keywords:
Title:
Structural basis for cooperative binding of Ribbon-Helix-Helix Omega repressor to inverted DNA heptad repeats
Biological Source:
Source Organism(s):
STREPTOCOCCUS PYOGENES (Taxon ID: 1314)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
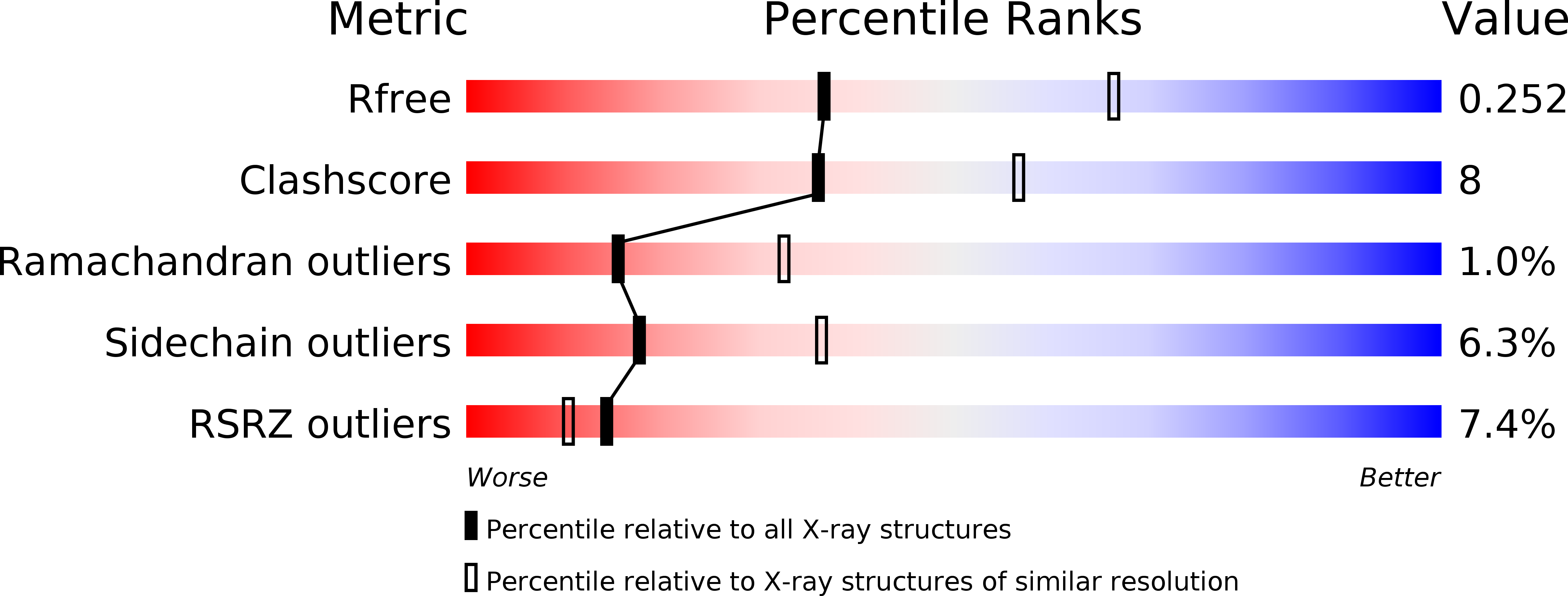
Resolution:
2.60 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1 21 1