Deposition Date
2005-03-18
Release Date
2006-01-17
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2BN4
Keywords:
Title:
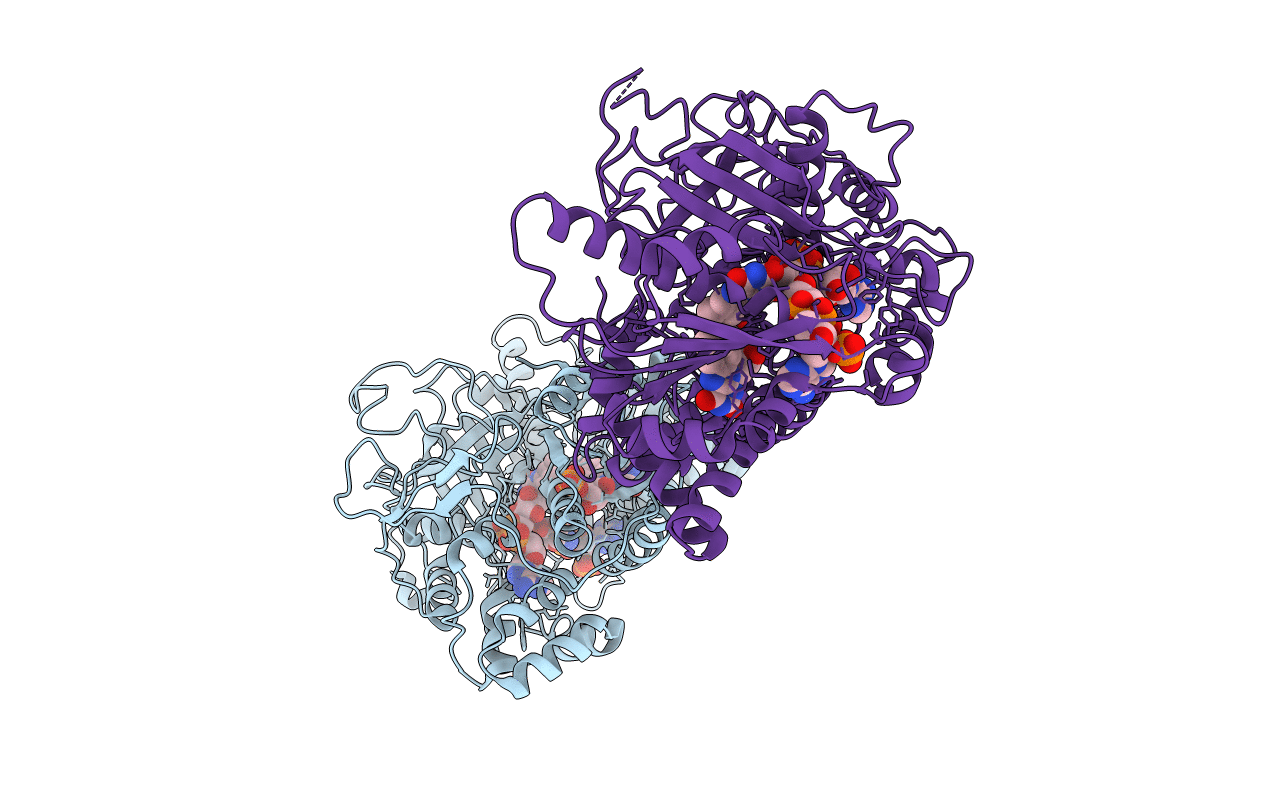
A second FMN-binding site in yeast NADPH-cytochrome P450 reductase suggests a novel mechanism of electron transfer by diflavin reductase
Biological Source:
Source Organism(s):
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
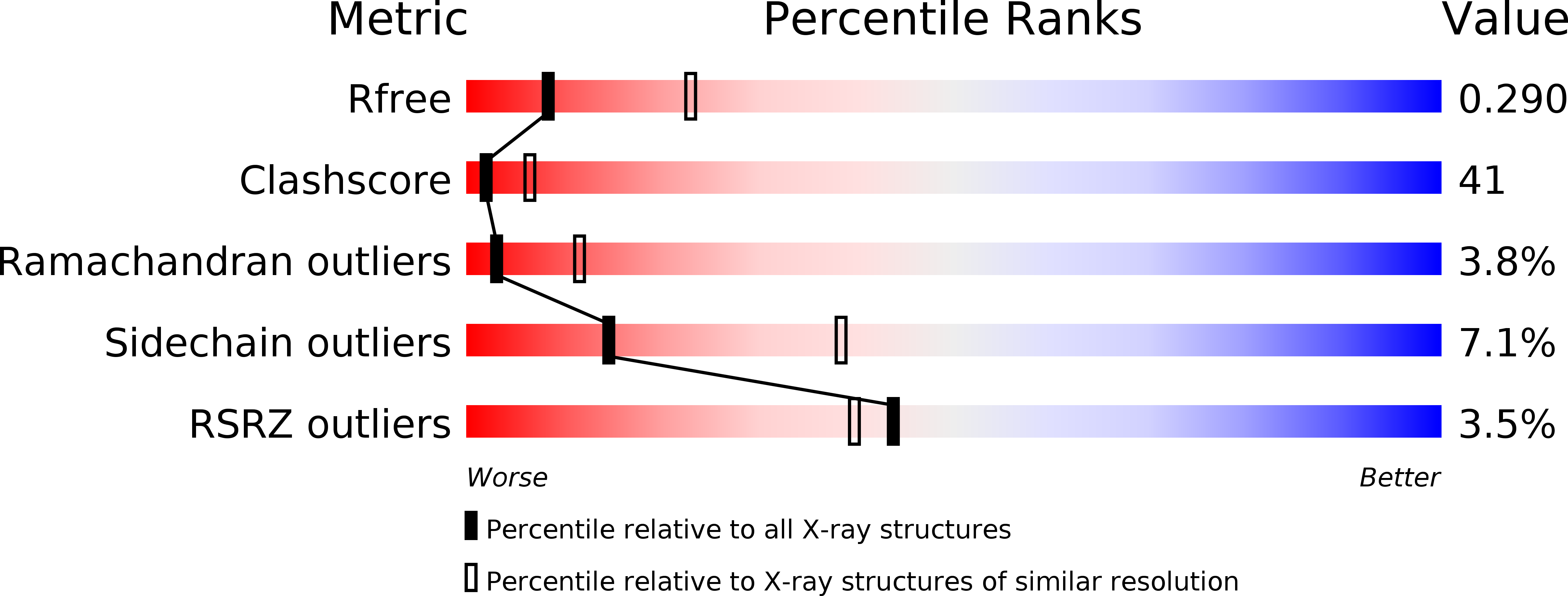
Resolution:
2.91 Å
R-Value Free:
0.30
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 21 21 21