Deposition Date
2005-03-10
Release Date
2005-05-19
Last Version Date
2023-12-13
Entry Detail
PDB ID:
2BMA
Keywords:
Title:
The crystal structure of Plasmodium falciparum glutamate dehydrogenase, a putative target for novel antimalarial drugs
Biological Source:
Source Organism(s):
PLASMODIUM FALCIPARUM (Taxon ID: 5833)
Expression System(s):
Method Details:
Experimental Method:
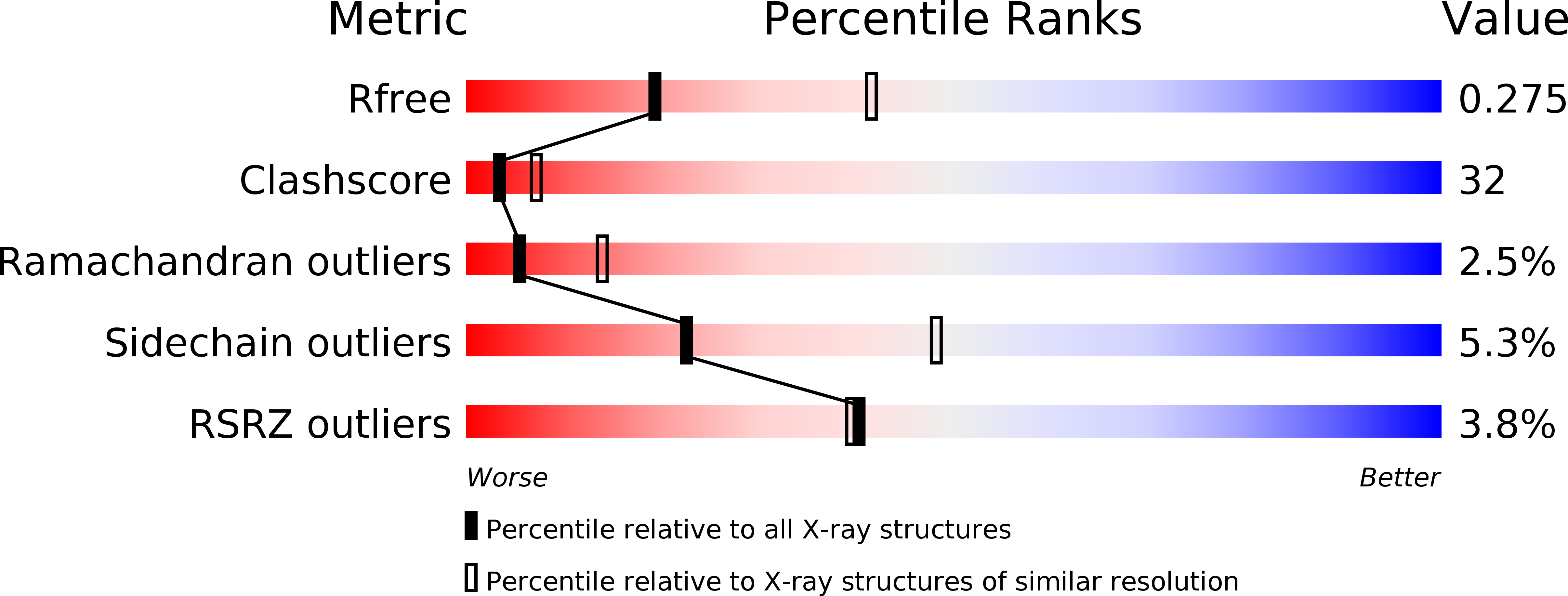
Resolution:
2.70 Å
R-Value Free:
0.27
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
C 1 2 1