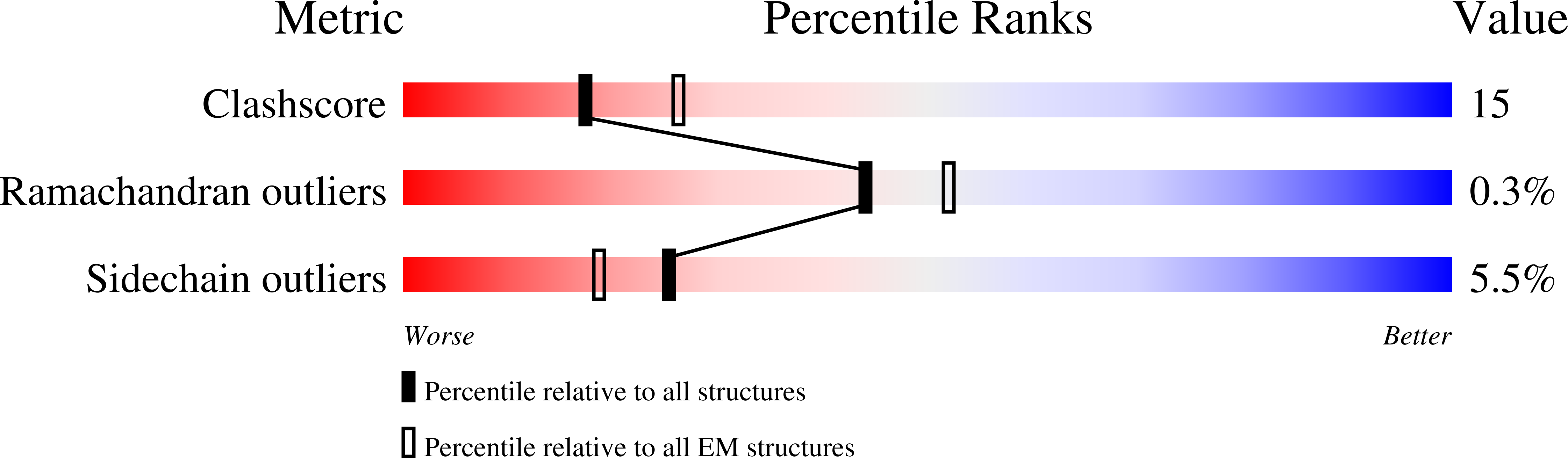
Deposition Date
2005-01-06
Release Date
2005-01-27
Last Version Date
2024-05-08
Entry Detail
PDB ID:
2BGZ
Keywords:
Title:
ATOMIC MODEL OF THE BACTERIAL FLAGELLAR BASED ON DOCKING AN X-RAY DERIVED HOOK STRUCTURE INTO AN EM MAP.
Biological Source:
Source Organism(s):
SALMONELLA TYPHIMURIUM (Taxon ID: 602)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
9.00 Å
Aggregation State:
2D ARRAY
Reconstruction Method:
CRYSTALLOGRAPHY