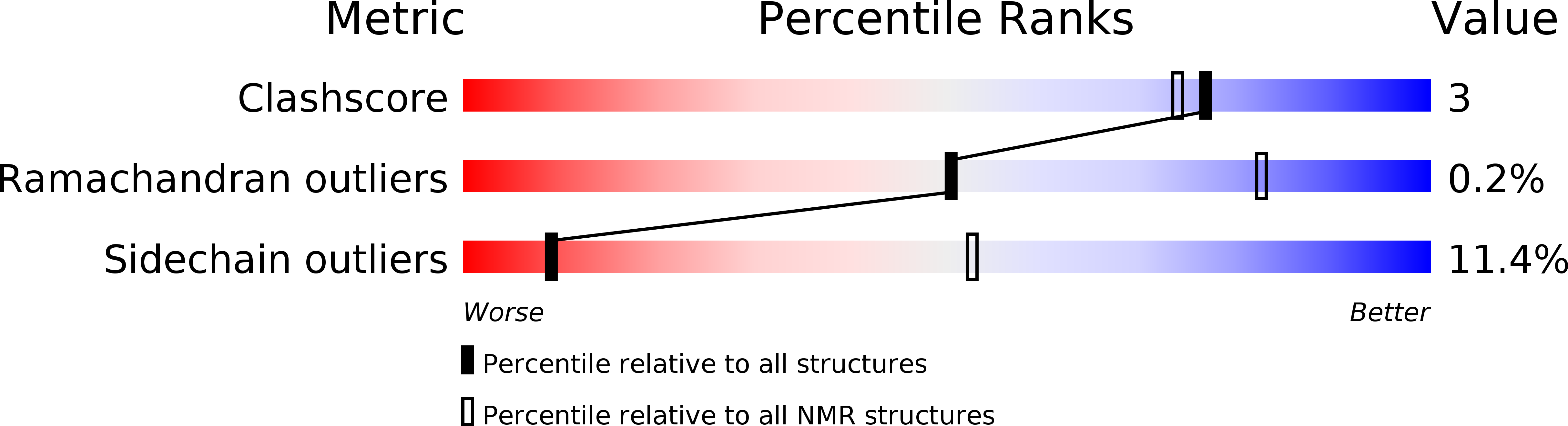Deposition Date
2004-12-22
Release Date
2005-08-31
Last Version Date
2024-05-15
Entry Detail
PDB ID:
2BGF
Keywords:
Title:
NMR structure of Lys48-linked di-ubiquitin using chemical shift perturbation data together with RDCs and 15N-relaxation data
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
10
Selection Criteria:
LOWEST ENERGY STRUCTURES OF LOWEST ENERGY CLUSTER