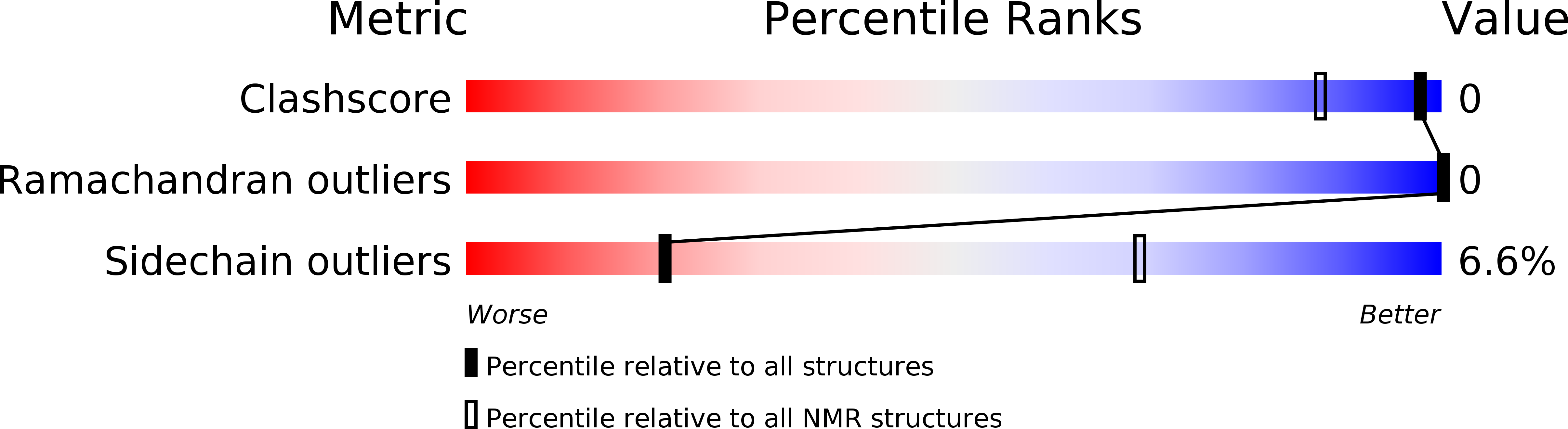
Deposition Date
2004-12-01
Release Date
2005-10-17
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2BEY
Keywords:
Title:
Solution Structure of a Novel C2 Symmetrical Bifunctional Bicyclic Inhibitor Based on SFTI-1
Biological Source:
Source Organism(s):
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Method Details: