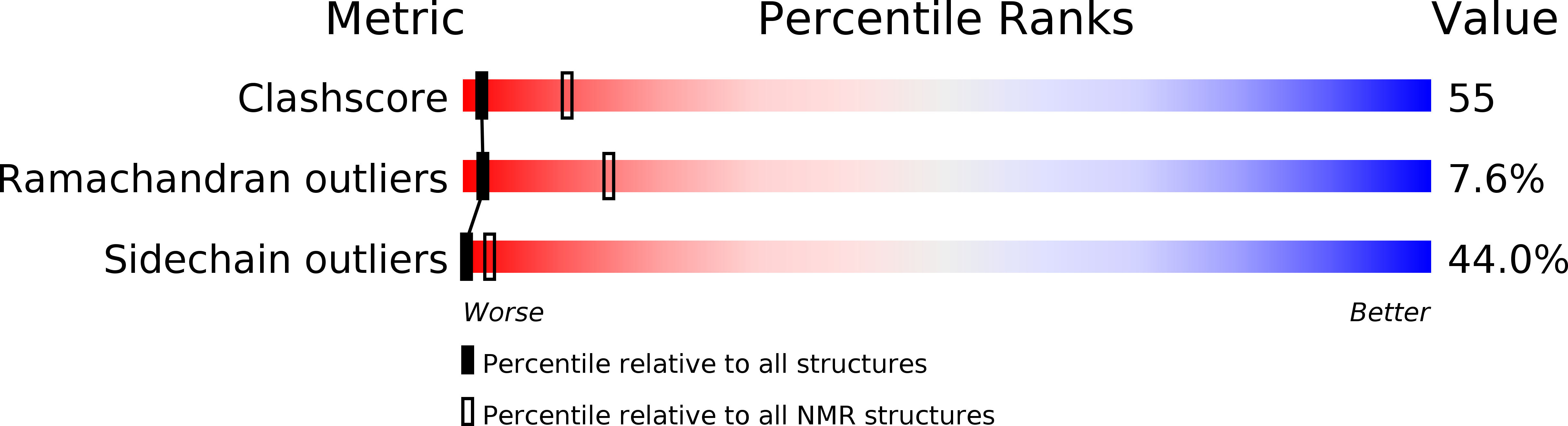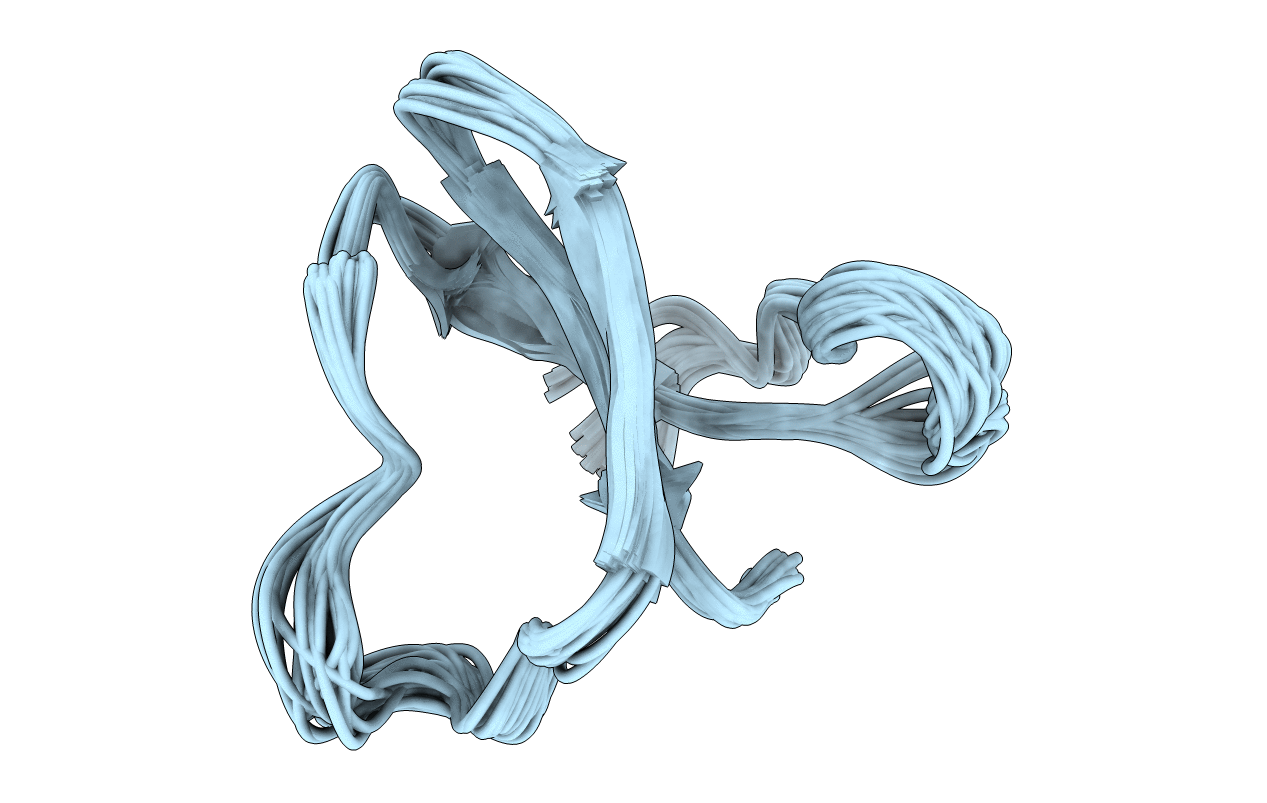
Deposition Date
1988-11-14
Release Date
1989-04-19
Last Version Date
2024-10-23
Entry Detail
PDB ID:
2BDS
Keywords:
Title:
DETERMINATION OF THE THREE-DIMENSIONAL SOLUTION STRUCTURE OF THE ANTIHYPERTENSIVE AND ANTIVIRAL PROTEIN BDS-I FROM THE SEA ANEMONE ANEMONIA SULCATA. A STUDY USING NUCLEAR MAGNETIC RESONANCE AND HYBRID DISTANCE GEOMETRY-DYNAMICAL SIMULATED ANNEALING
Biological Source:
Source Organism(s):
Anemonia sulcata (Taxon ID: 6108)
Method Details:
Experimental Method:
Conformers Submitted:
42