Deposition Date
1995-07-03
Release Date
1996-08-01
Last Version Date
2024-02-14
Entry Detail
PDB ID:
2AFN
Keywords:
Title:
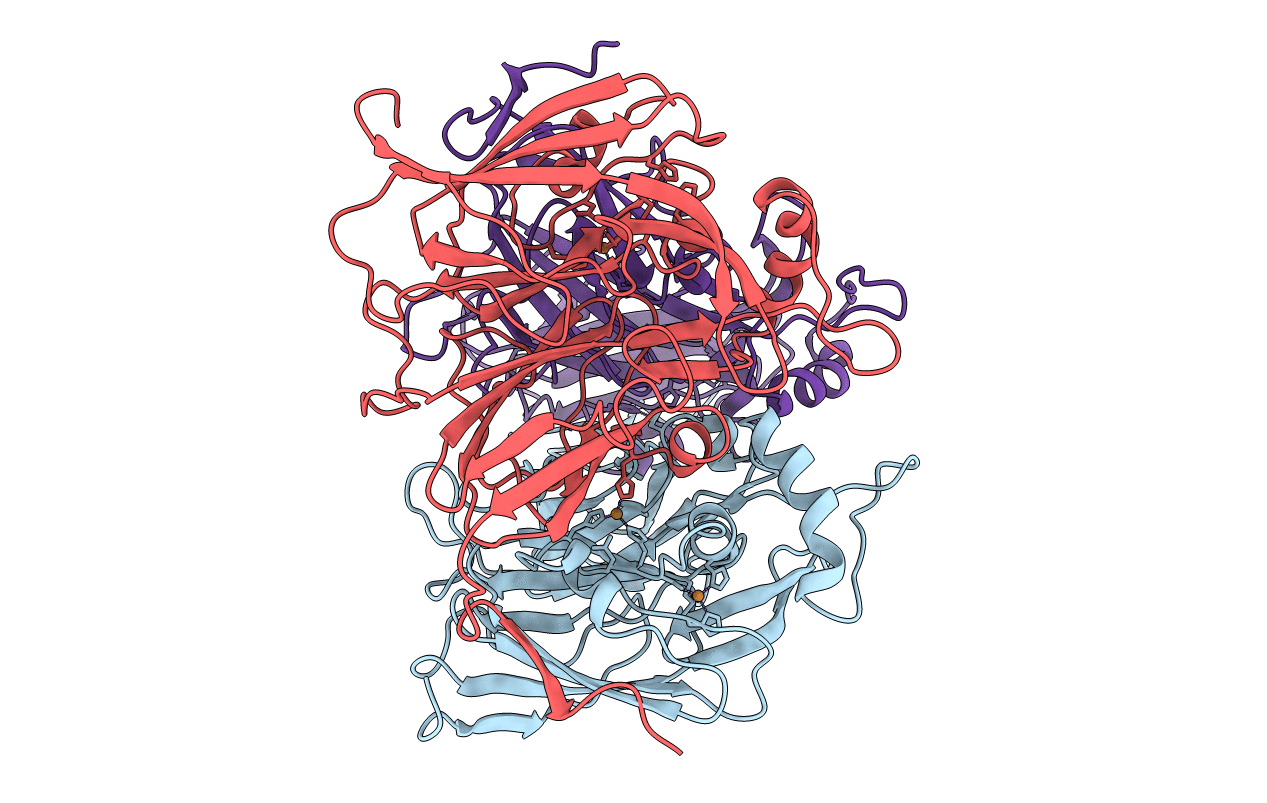
STRUCTURE OF ALCALIGENES FAECALIS NITRITE REDUCTASE AND A COPPER SITE MUTANT, M150E, THAT CONTAINS ZINC
Biological Source:
Source Organism(s):
Alcaligenes faecalis (Taxon ID: 511)
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.22
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 21 21 21