Deposition Date
2005-07-23
Release Date
2005-12-20
Last Version Date
2024-11-20
Entry Detail
PDB ID:
2AEP
Keywords:
Title:
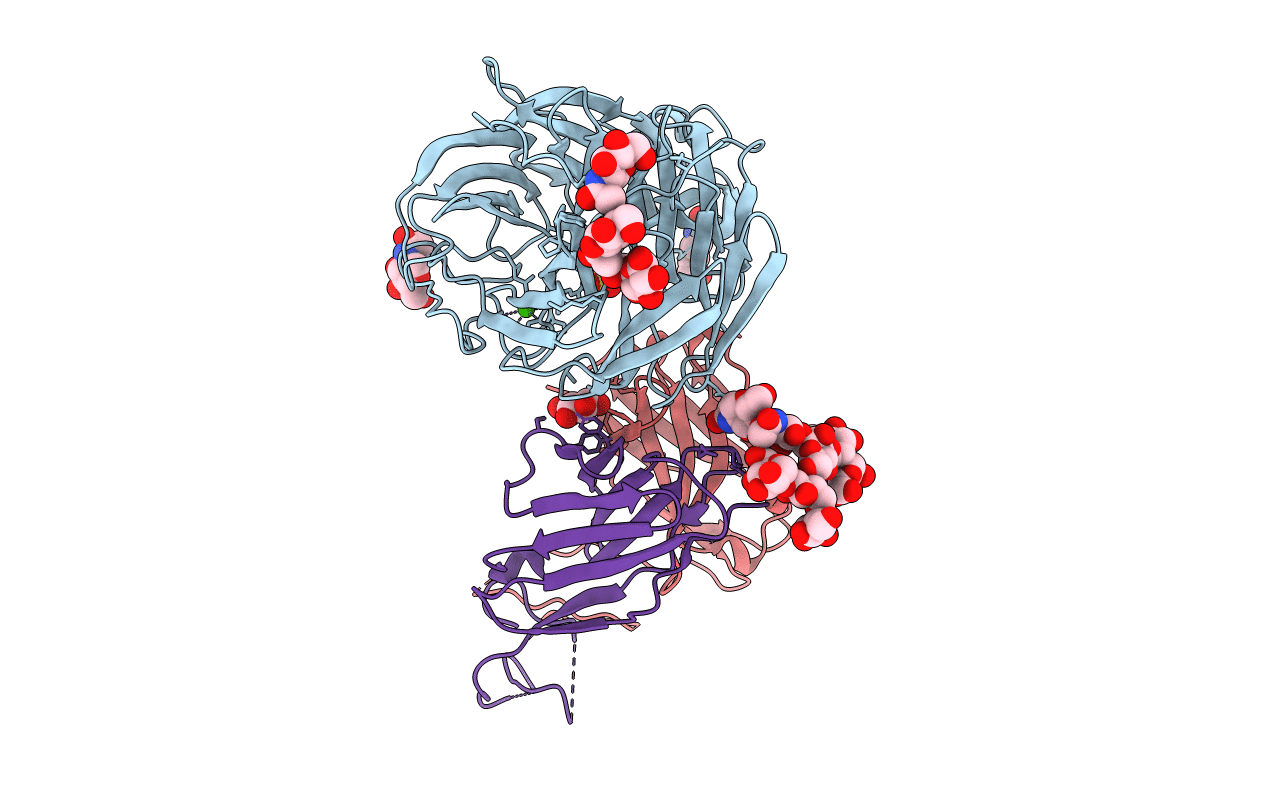
An epidemiologically significant epitope of a 1998 influenza virus neuraminidase forms a highly hydrated interface in the NA-antibody complex.
Biological Source:
Source Organism(s):
Influenza A virus (Taxon ID: 228928)
Mus musculus (Taxon ID: 10090)
Mus musculus (Taxon ID: 10090)
Method Details:
Experimental Method:
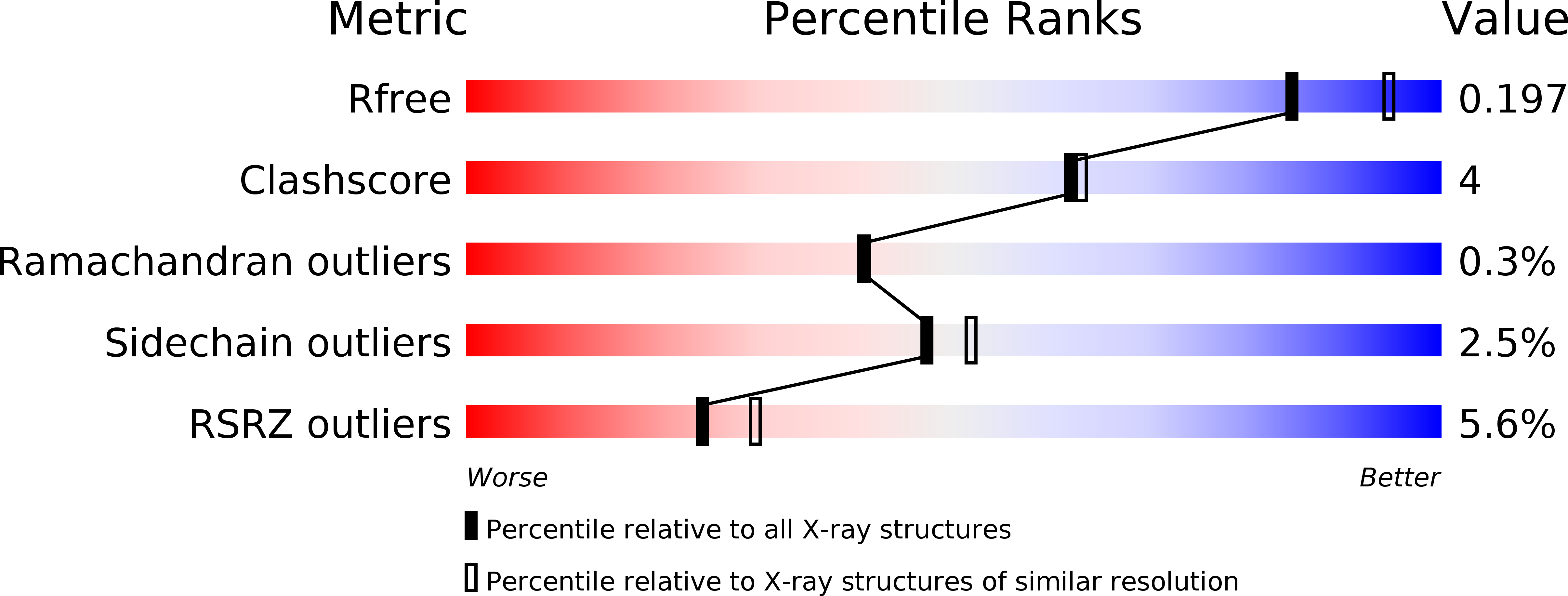
Resolution:
2.10 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 4 21 2