Abstact
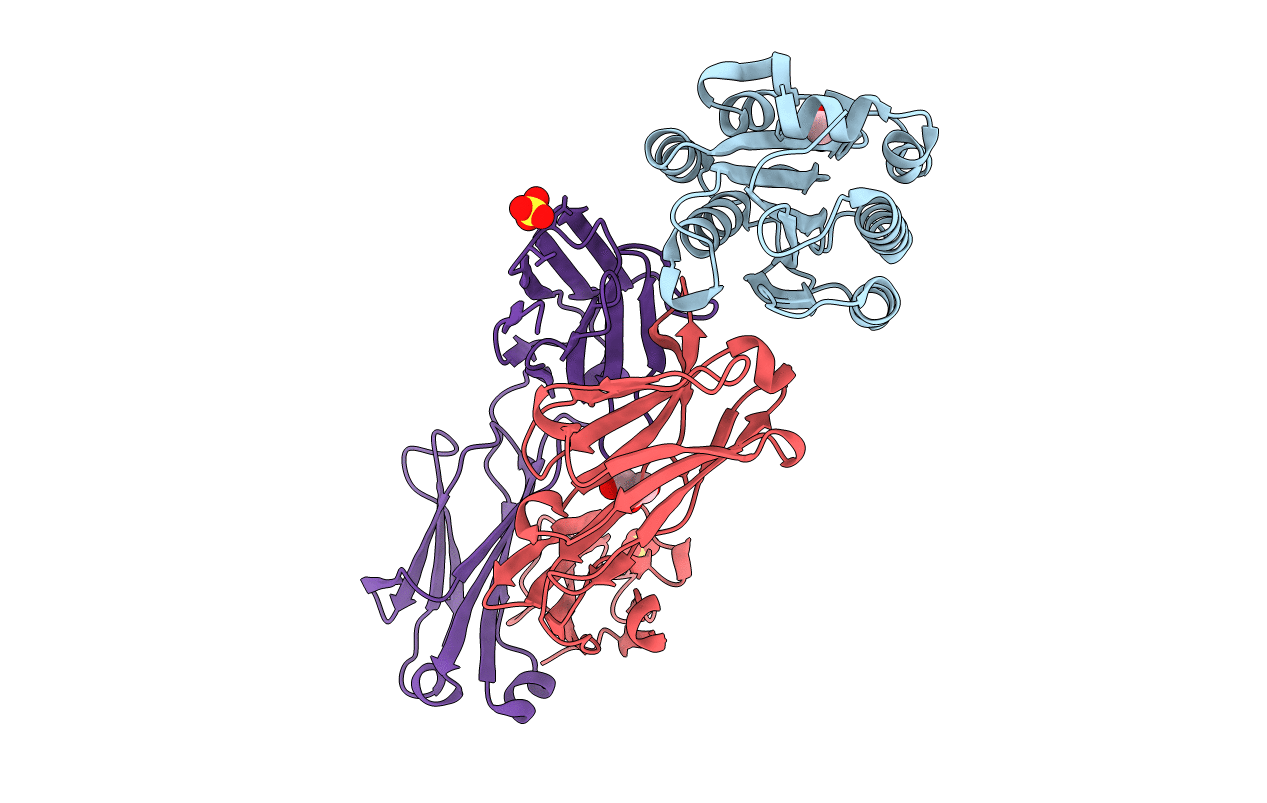
The antithrombotic monoclonal antibody 82D6A3 is directed against amino acids Arg-963, Pro-981, Asp-1009, Arg-1016, Ser-1020, Met-1022, and His-1023 of the von Willebrand factor A3-domain (Vanhoorelbeke, K., Depraetere, H., Romijn, R. A., Huizinga, E., De Maeyer, M., and Deckmyn, H. (2003) J. Biol. Chem. 278, 37815-37821). By this, it potently inhibits the interaction of von Willebrand factor to collagens, which is a prerequisite for blood platelet adhesion to the injured vessel wall at sites of high shear. To fully understand the mode of action of 82D6A3 at the molecular level, we resolved its crystal structure in complex with the A3-domain and fine mapped its paratope by construction and characterization of 13 mutants. The paratope predominantly consists of two short sequences in the heavy chain CDR1 (Asn-31 and Tyr-32) and CDR3 (Asp-99, Pro-101, Tyr-102 and Tyr-103), forming one patch on the surface of the antibody. Trp-50 of the heavy and His-49 of the light chain, both situated adjacent to the patch, play ancillary roles in antigen binding. The crystal structure furthermore confirms the epitope location, which largely overlaps with the collagen binding site deduced from mutagenesis of the A3-domain (Romijn, R. A., Westein, E., Bouma, B., Schiphorst, M. E., Sixma, J. J., Lenting, P. J., and Huizinga, E. G. (2003) J. Biol. Chem. 278, 15035-15039). We herewith further consolidate the location of the collagen binding site and reveal that the potent action of the antibody is due to direct competition for the same interaction site. This information allows the design of a paratope-mimicking peptide with antithrombotic properties.