Deposition Date
2005-07-01
Release Date
2006-06-06
Last Version Date
2024-05-22
Entry Detail
PDB ID:
2A63
Keywords:
Title:
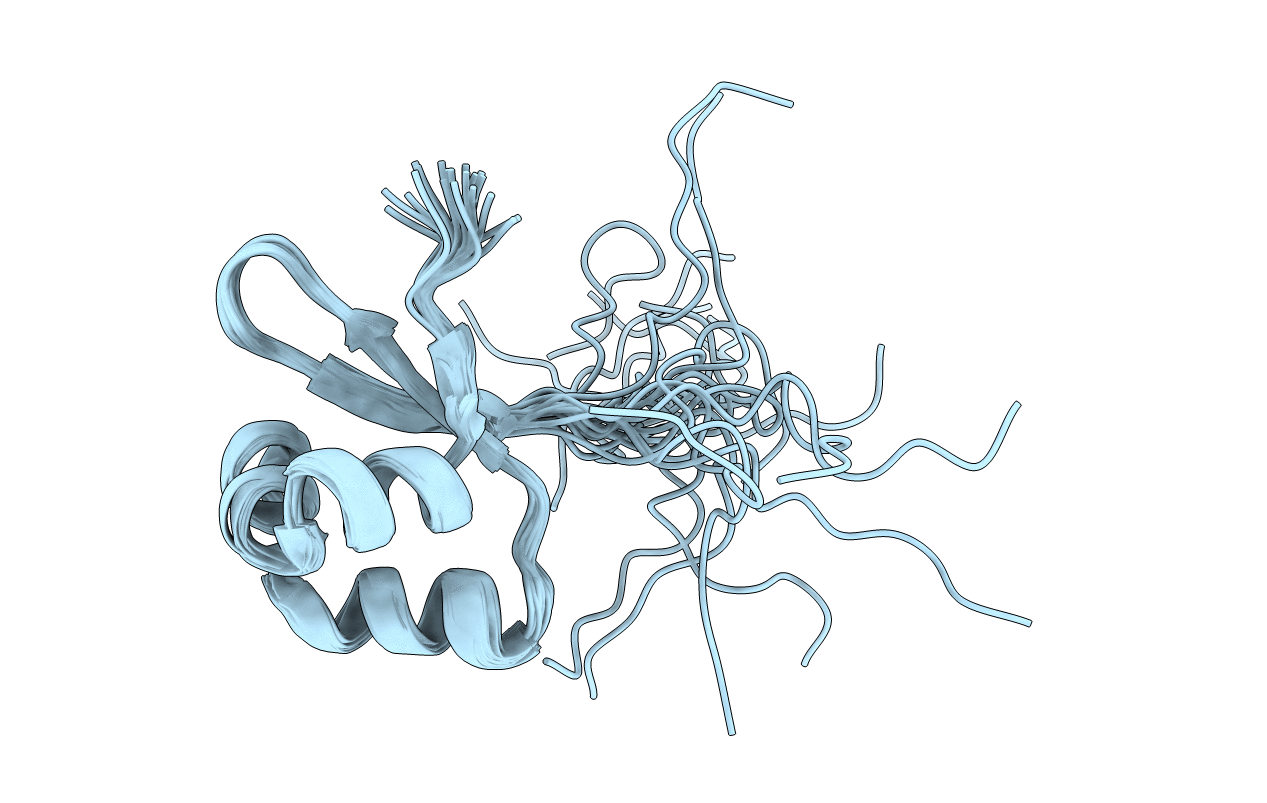
Solution structure of a stably monomeric mutant of lambda Cro produced by substitutions in the ball-and-socket interface
Biological Source:
Source Organism(s):
Enterobacteria phage lambda (Taxon ID: 10710)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
20
Selection Criteria:
all structures compatible with experimental restraints; structures chosen had the lowest energies and/or best agreement to J(NHB) data not used in explicit restraints