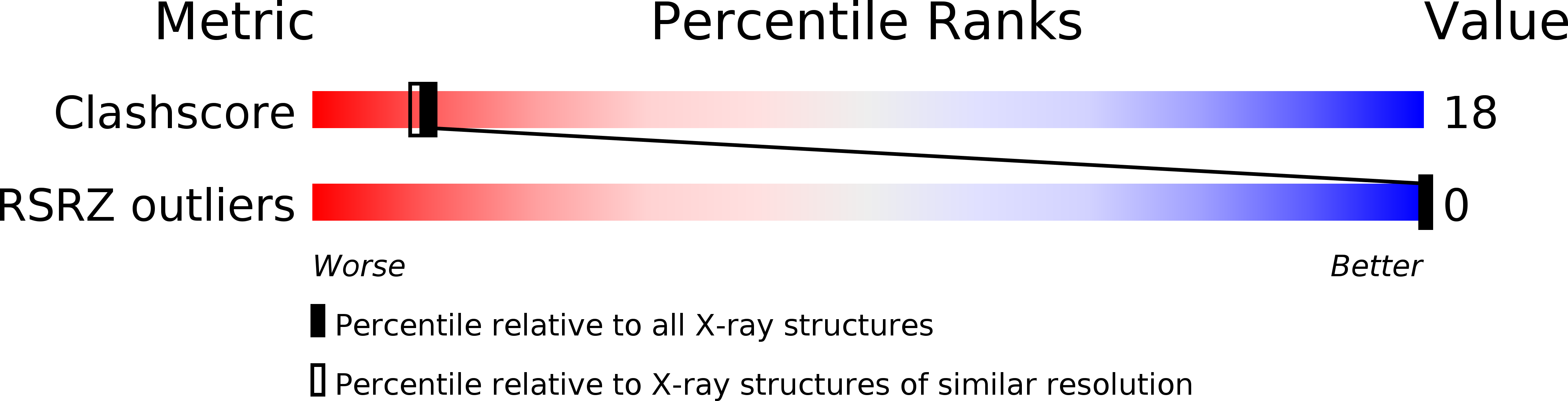Deposition Date
1996-10-10
Release Date
1996-12-17
Last Version Date
2024-04-03
Entry Detail
PDB ID:
289D
Keywords:
Title:
TARGETING THE MINOR GROOVE OF DNA: CRYSTAL STRUCTURES OF TWO COMPLEXES BETWEEN FURAN DERIVATIVES OF BERENIL AND THE DNA DODECAMER D(CGCGAATTCGCG)2
Method Details:
Experimental Method:
Resolution:
2.20 Å
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21