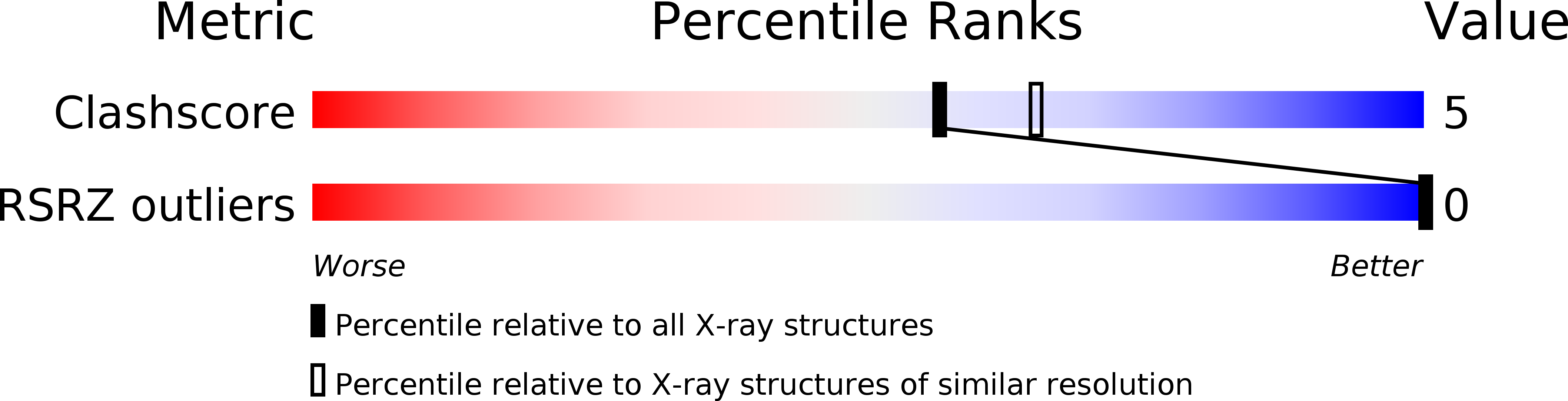
Deposition Date
1995-08-08
Release Date
1995-11-11
Last Version Date
2024-02-14
Entry Detail
PDB ID:
227D
Keywords:
Title:
A CRYSTALLOGRAPHIC AND SPECTROSCOPIC STUDY OF THE COMPLEX BETWEEN D(CGCGAATTCGCG)2 AND 2,5-BIS(4-GUANYLPHENYL)FURAN, AN ANALOGUE OF BERENIL. STRUCTURAL ORIGINS OF ENHANCED DNA-BINDING AFFINITY
Method Details:
Experimental Method:
Resolution:
2.20 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21