Deposition Date
2005-05-16
Release Date
2005-08-09
Last Version Date
2023-08-23
Entry Detail
PDB ID:
1ZP4
Keywords:
Title:
Glu28Gln mutant of E. coli Methylenetetrahydrofolate Reductase (oxidized) complex with Methyltetrahydrofolate
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 562)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.85 Å
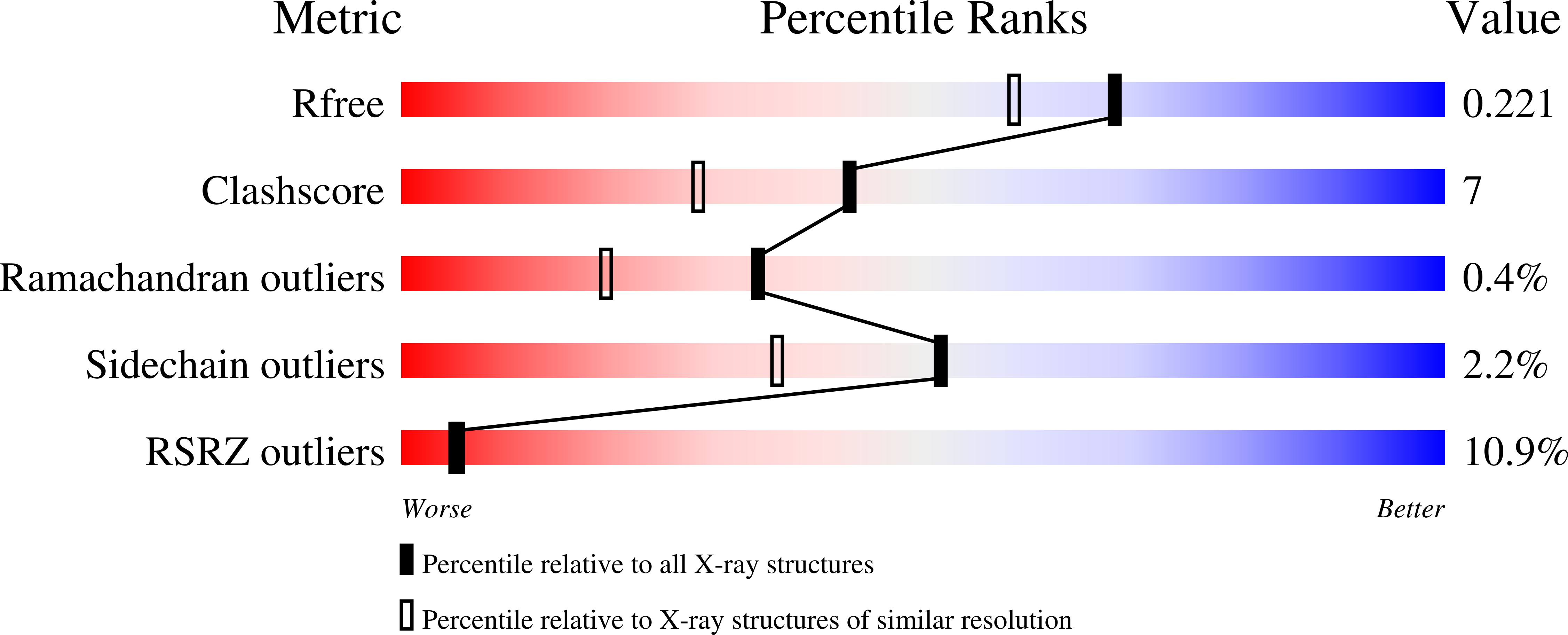
R-Value Free:
0.23
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
C 1 2 1