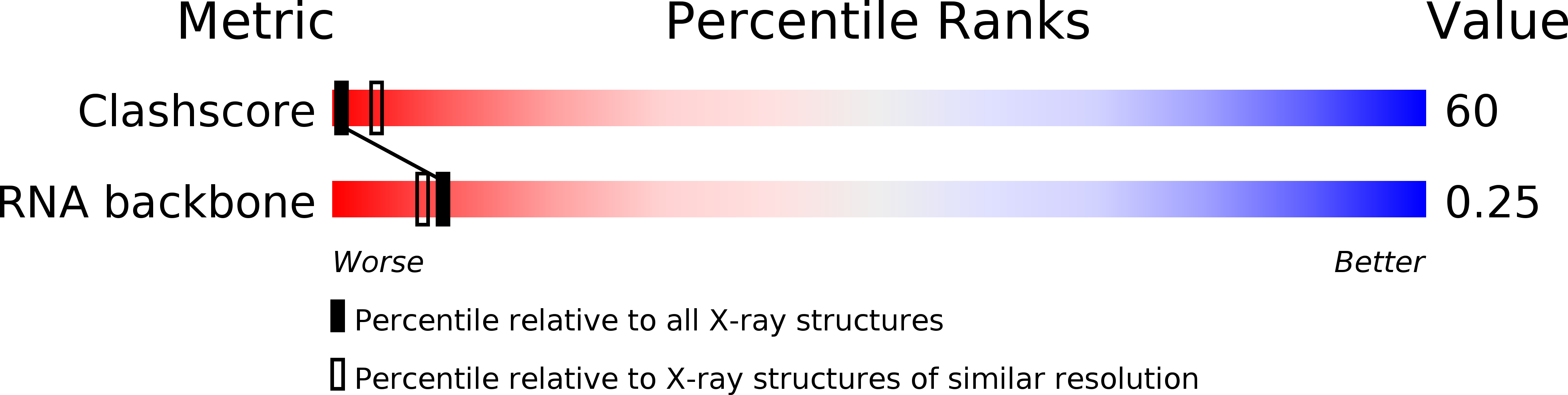
Deposition Date
1986-05-16
Release Date
1986-07-14
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1TRA
Keywords:
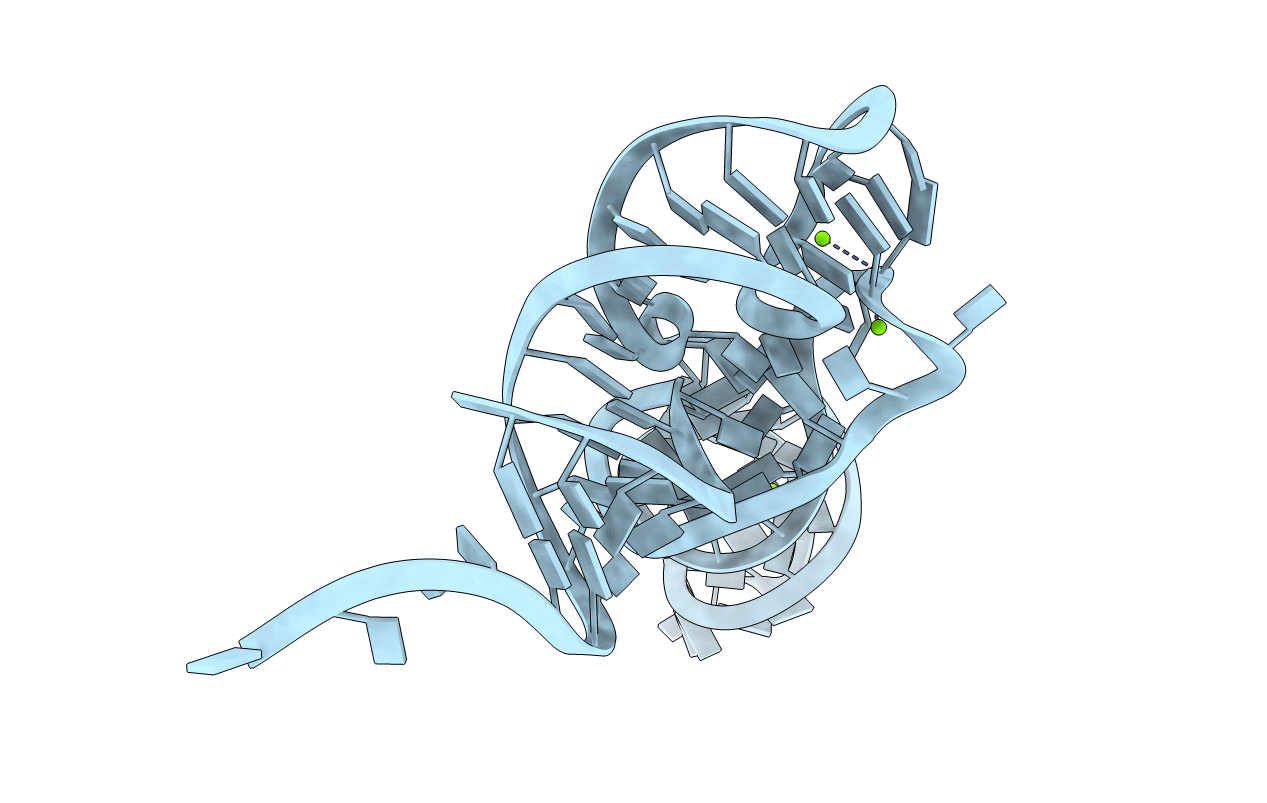
Title:
RESTRAINED REFINEMENT OF THE MONOCLINIC FORM OF YEAST PHENYLALANINE TRANSFER RNA. TEMPERATURE FACTORS AND DYNAMICS, COORDINATED WATERS, AND BASE-PAIR PROPELLER TWIST ANGLES
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Method Details:
Experimental Method:
Resolution:
3.00 Å
R-Value Observed:
0.16
Space Group:
P 1 21 1