Deposition Date
2003-08-27
Release Date
2003-11-11
Last Version Date
2023-08-16
Entry Detail
PDB ID:
1QVF
Keywords:
Title:
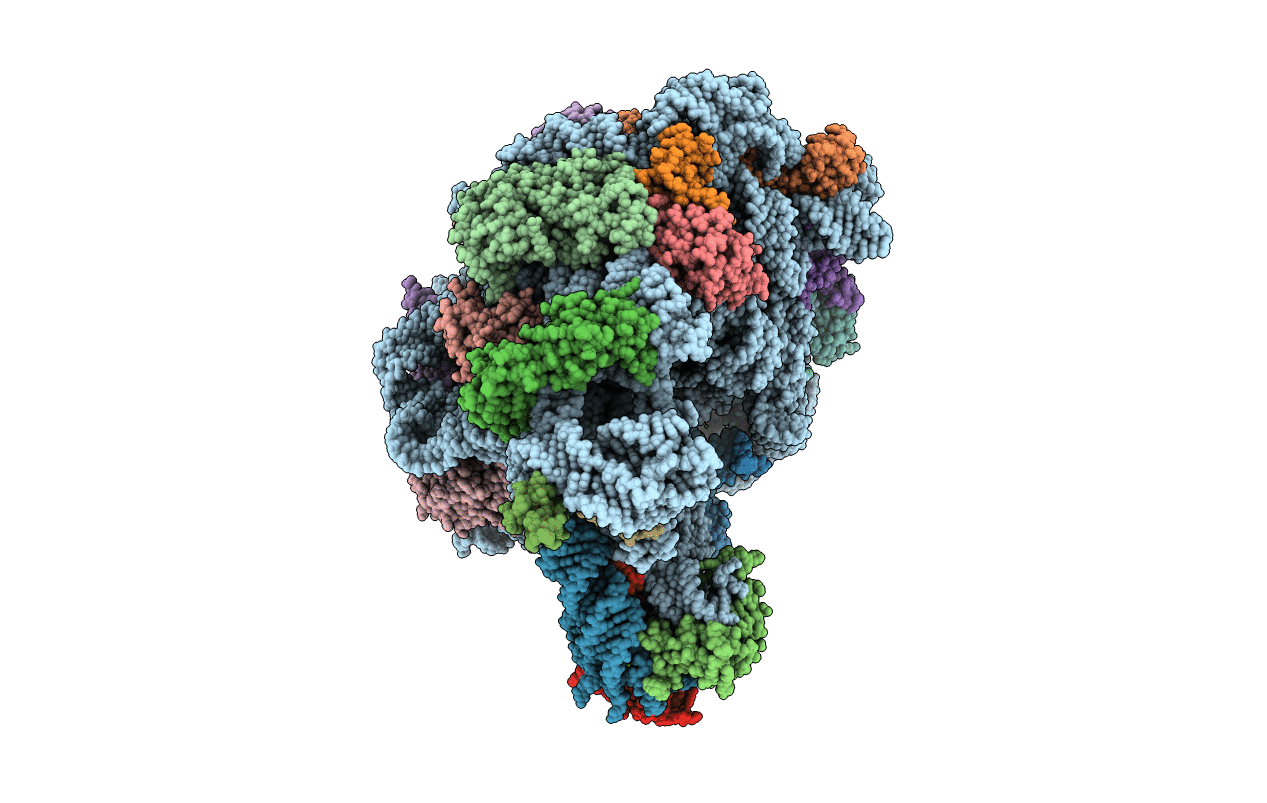
Structure of a deacylated tRNA minihelix bound to the E site of the large ribosomal subunit of Haloarcula marismortui
Biological Source:
Source Organism(s):
Haloarcula marismortui (Taxon ID: 2238)
Method Details:
Experimental Method:
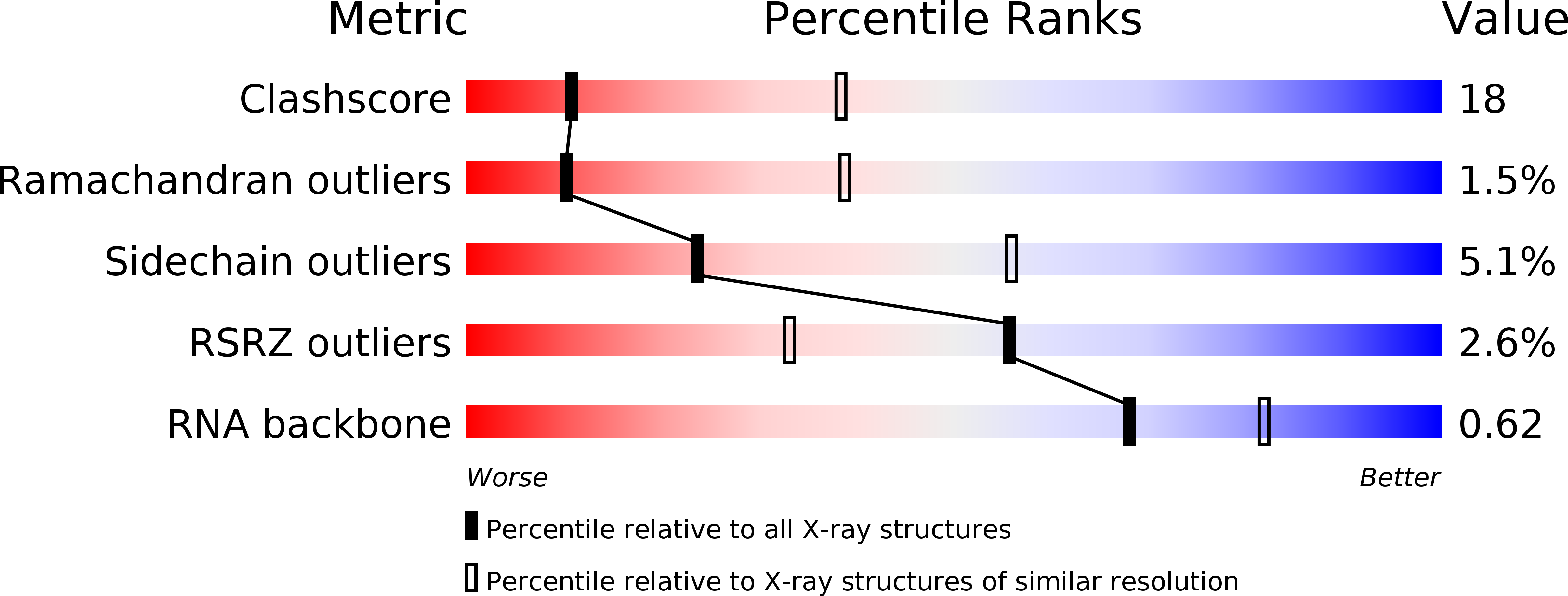
Resolution:
3.10 Å
R-Value Free:
0.23
R-Value Work:
0.19
Space Group:
C 2 2 21