Deposition Date
2002-07-03
Release Date
2003-04-22
Last Version Date
2023-10-25
Entry Detail
PDB ID:
1M4N
Keywords:
Title:
CRYSTAL STRUCTURE OF APPLE ACC SYNTHASE IN COMPLEX WITH [2-(AMINO-OXY)ETHYL](5'-DEOXYADENOSIN-5'-YL)(METHYL)SULFONIUM
Biological Source:
Source Organism(s):
Malus x domestica (Taxon ID: 3750)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.01 Å
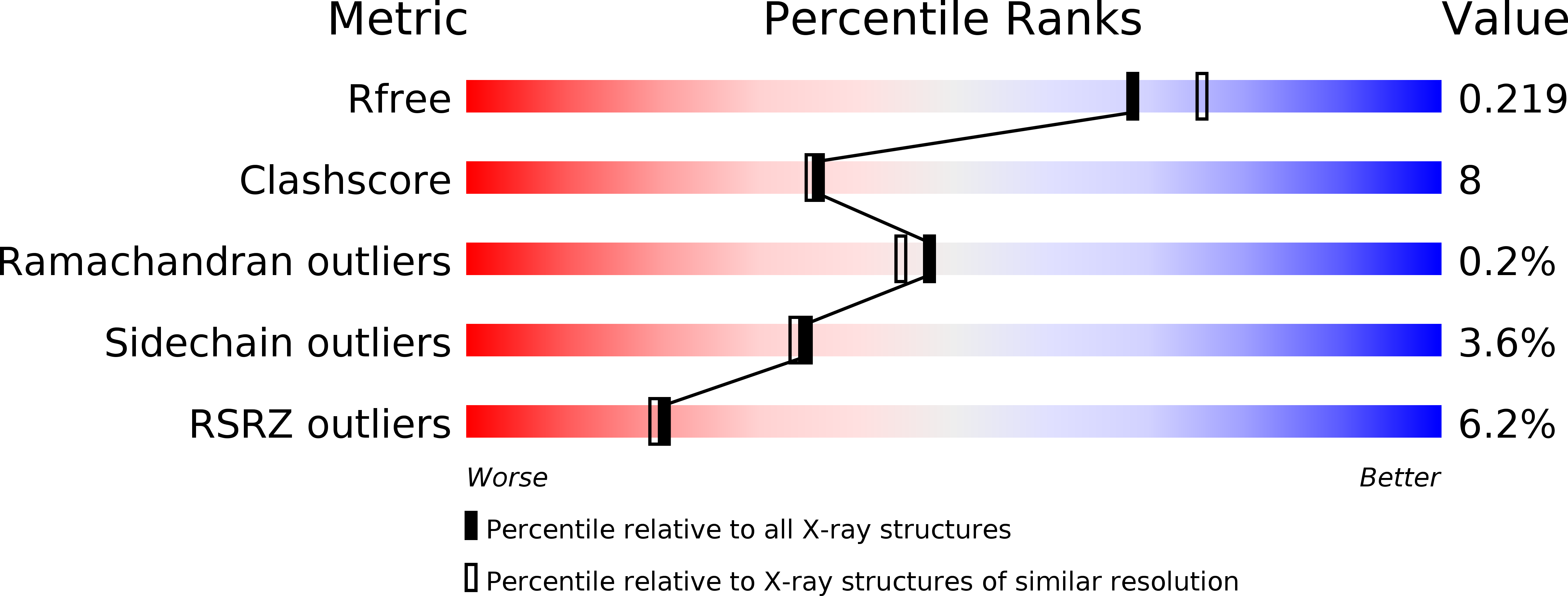
R-Value Free:
0.23
R-Value Work:
0.19
Space Group:
C 1 2 1