Deposition Date
2003-03-11
Release Date
2003-06-19
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1HKU
Keywords:
Title:
CtBP/BARS: a dual-function protein involved in transcription corepression and Golgi membrane fission
Biological Source:
Source Organism:
RATTUS NORVEGICUS (Taxon ID: 10116)
Host Organism:
Method Details:
Experimental Method:
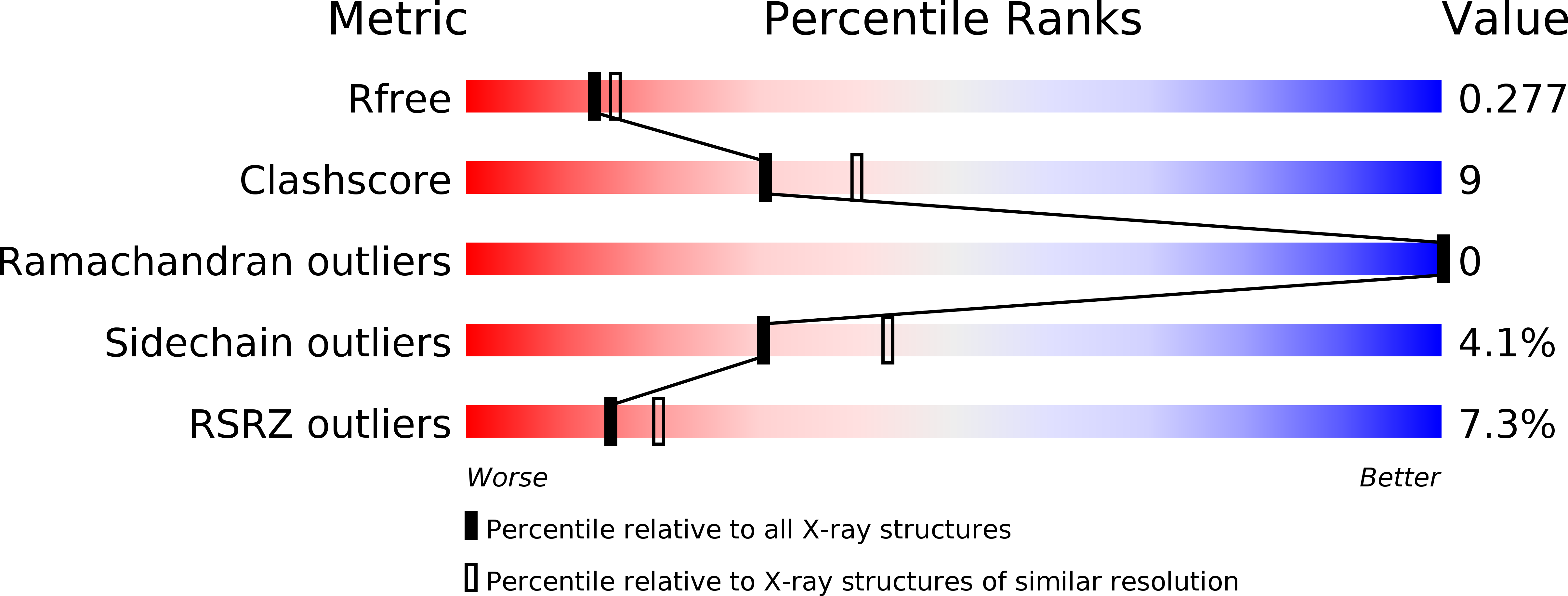
Resolution:
2.30 Å
R-Value Free:
0.28
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 64 2 2