Deposition Date
1993-02-24
Release Date
1993-10-31
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1GUH
Keywords:
Title:
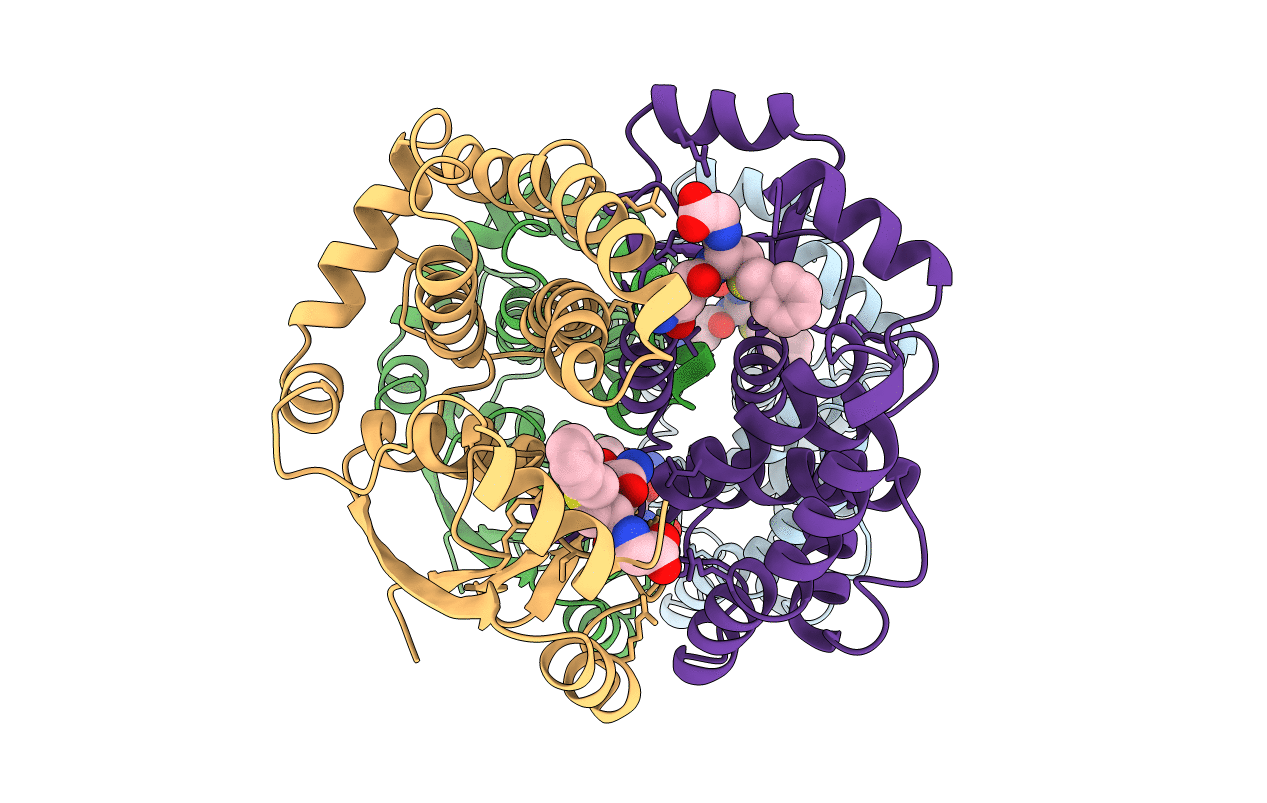
Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the MU and PI class enzymes
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
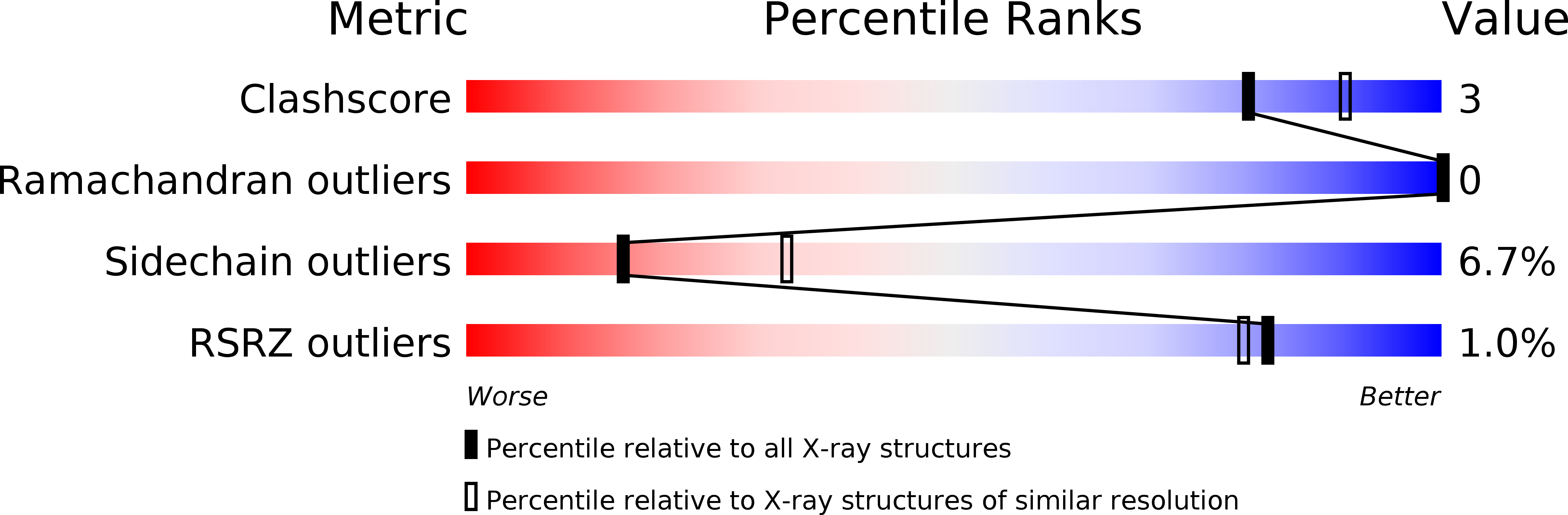
Resolution:
2.60 Å
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1