Deposition Date
2002-01-15
Release Date
2002-04-05
Last Version Date
2023-12-13
Entry Detail
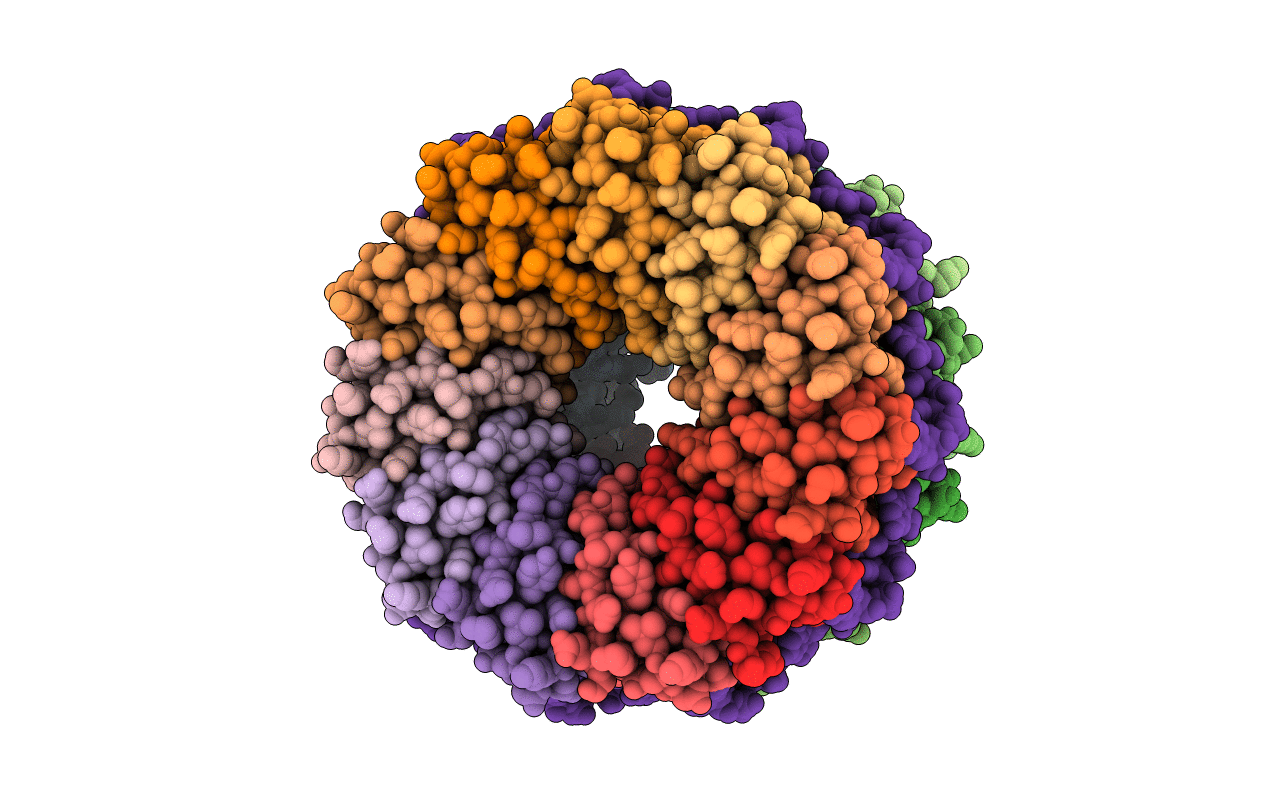
PDB ID:
1GTF
Keywords:
Title:
The structure of the trp RNA-binding attenuation protein (TRAP) bound to a 53-nucleotide RNA molecule containing GAGUU repeats
Biological Source:
Source Organism(s):
BACILLUS STEAROTHERMOPHILUS (Taxon ID: 1422)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.75 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1


