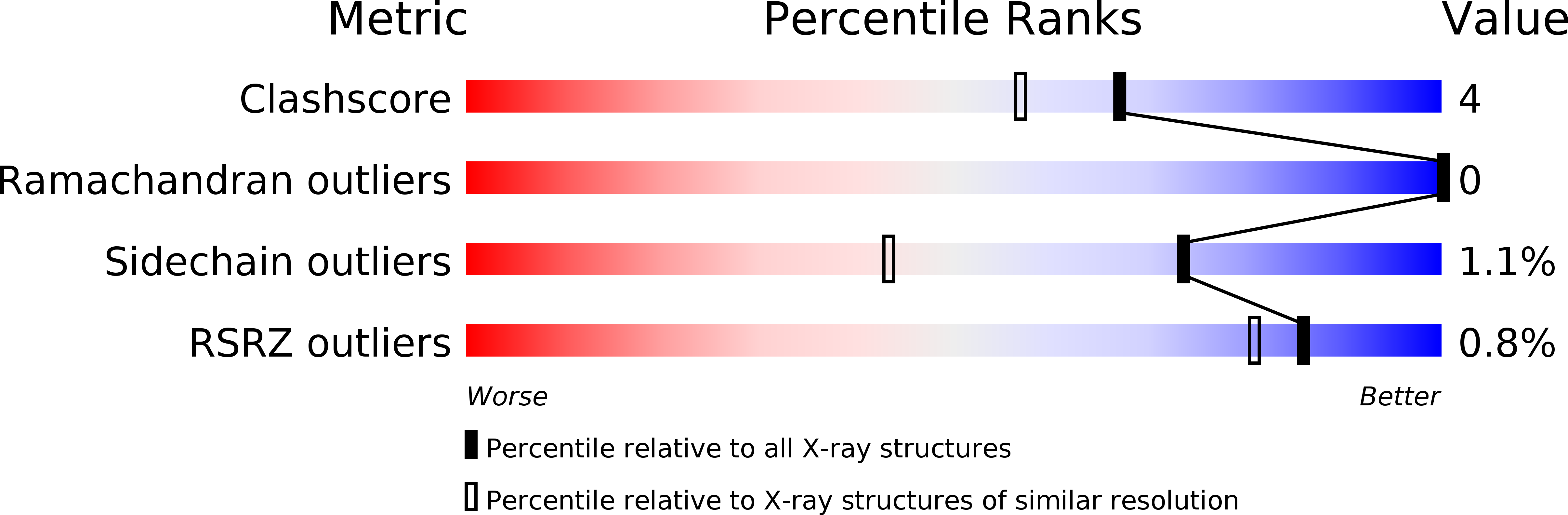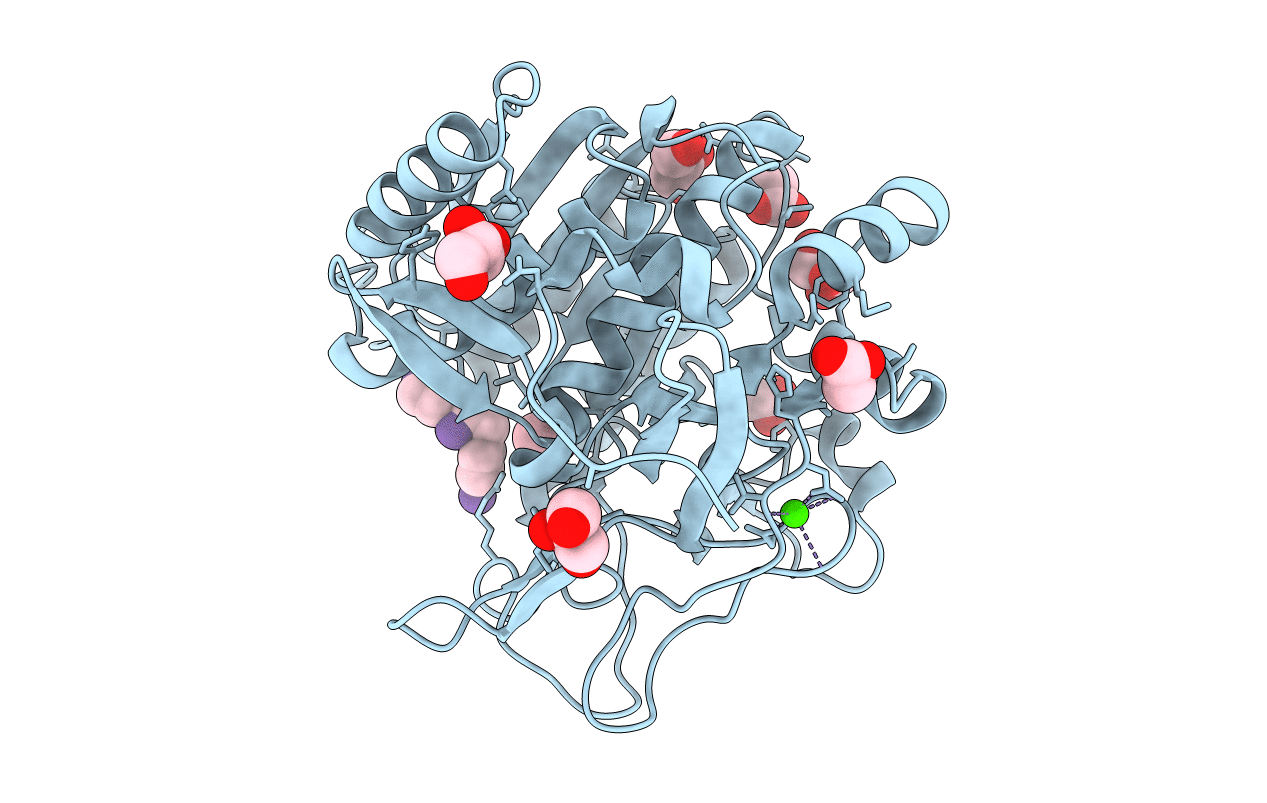
Deposition Date
2000-11-29
Release Date
2000-12-13
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1GA6
Keywords:
Title:
CRYSTAL STRUCTURE ANALYSIS OF PSCP (PSEUDOMONAS SERINE-CARBOXYL PROTEINASE) COMPLEXED WITH A FRAGMENT OF TYROSTATIN (THIS ENZYME RENAMED "SEDOLISIN" IN 2003)
Biological Source:
Source Organism:
Pseudomonas sp. (Taxon ID: 306)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.00 Å
R-Value Free:
0.13
R-Value Work:
0.12
R-Value Observed:
0.09
Space Group:
P 62