Deposition Date
1999-06-05
Release Date
1999-06-18
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1CO6
Keywords:
Title:
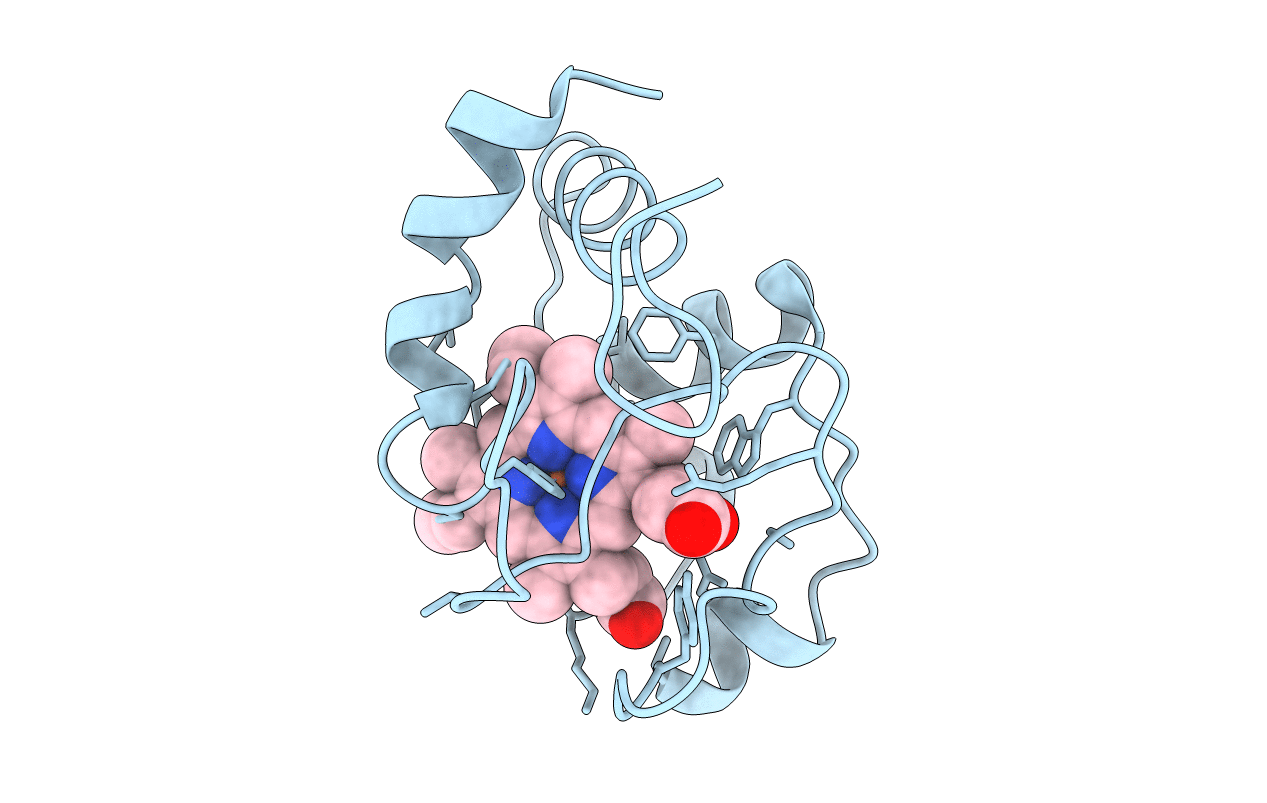
CRYSTAL STRUCTURE OF FERROCYTOCHROME C2 FROM RHODOPSEUDOMONAS VIRIDIS
Biological Source:
Source Organism(s):
Blastochloris viridis (Taxon ID: 1079)
Method Details: