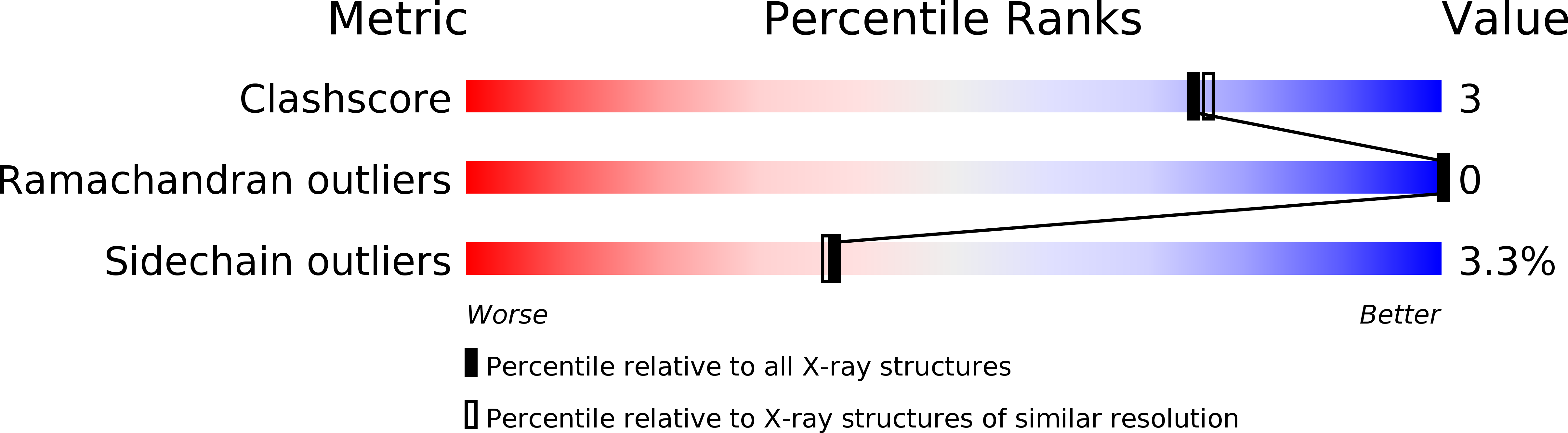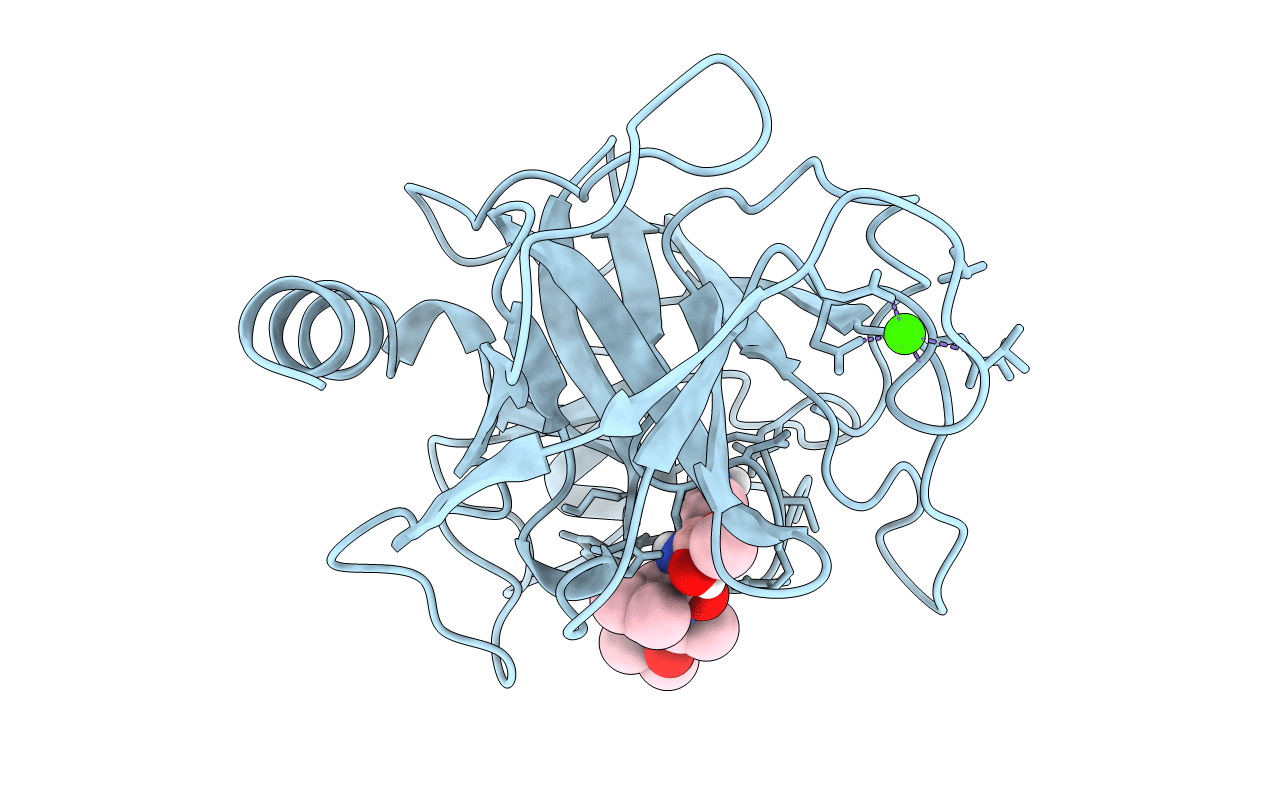
Deposition Date
1995-05-17
Release Date
1995-10-15
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1BTZ
Keywords:
Title:
Episelection: novel KI ~nanomolar inhibitors of serine proteases selected by binding or chemistry on an enzyme surface
Biological Source:
Source Organism:
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 21 21 21