Deposition Date
2005-06-09
Release Date
2005-09-27
Last Version Date
2023-08-23
Entry Detail
PDB ID:
1ZY0
Keywords:
Title:
X-ray structure of peptide deformylase from Arabidopsis thaliana (AtPDF1A); crystals grown in PEG-6000
Biological Source:
Source Organism:
Arabidopsis thaliana (Taxon ID: 3702)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.90 Å
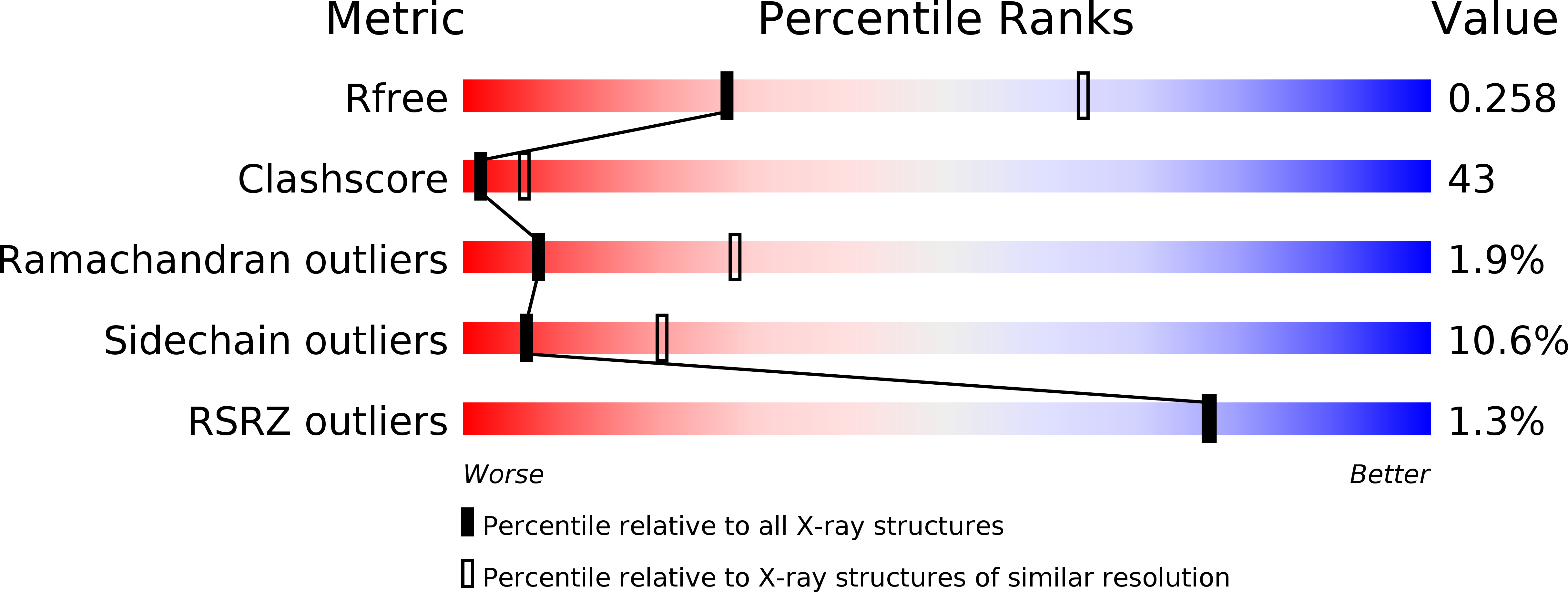
R-Value Free:
0.28
R-Value Work:
0.24
Space Group:
P 21 21 21