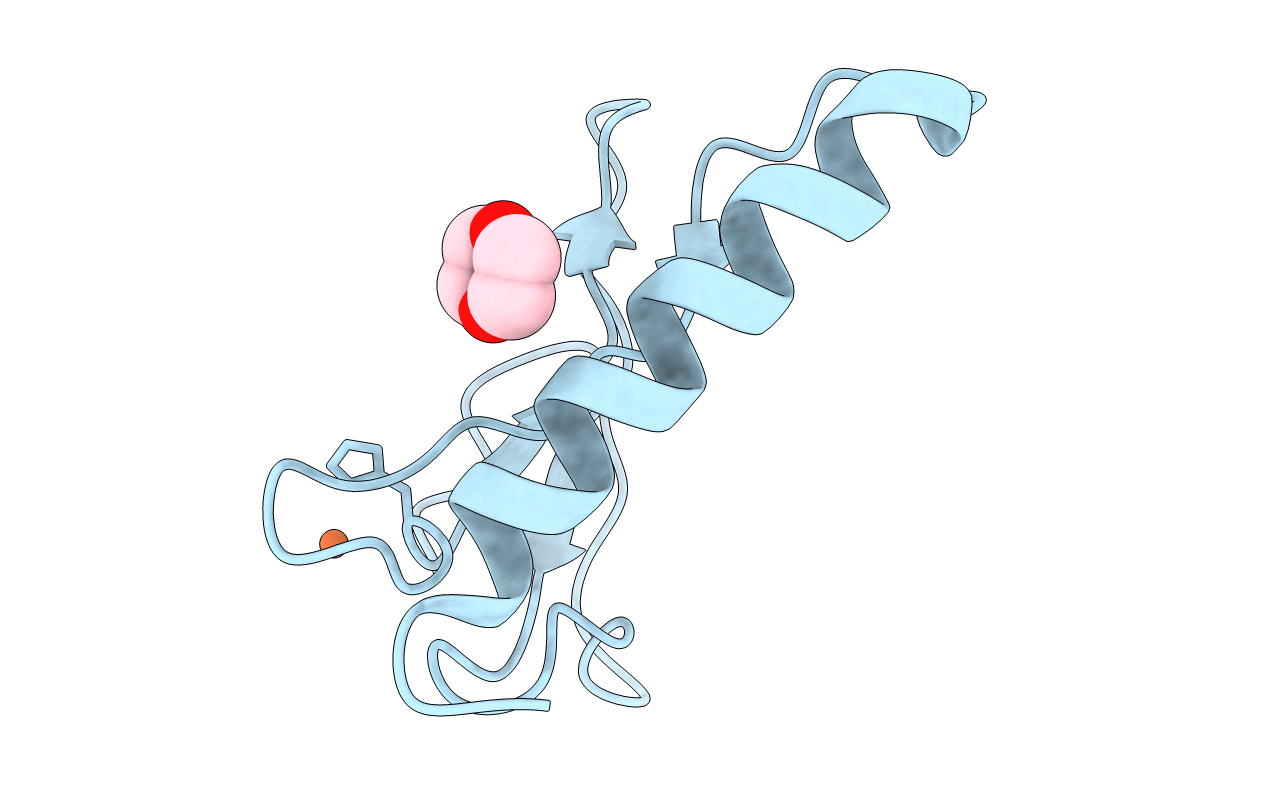
Deposition Date
2005-05-26
Release Date
2005-06-28
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1ZT5
Keywords:
Title:
C-terminal domain of Insulin-like Growth Factor Binding Protein-1 isolated from human amniotic fluid complexed with Iron(II)
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
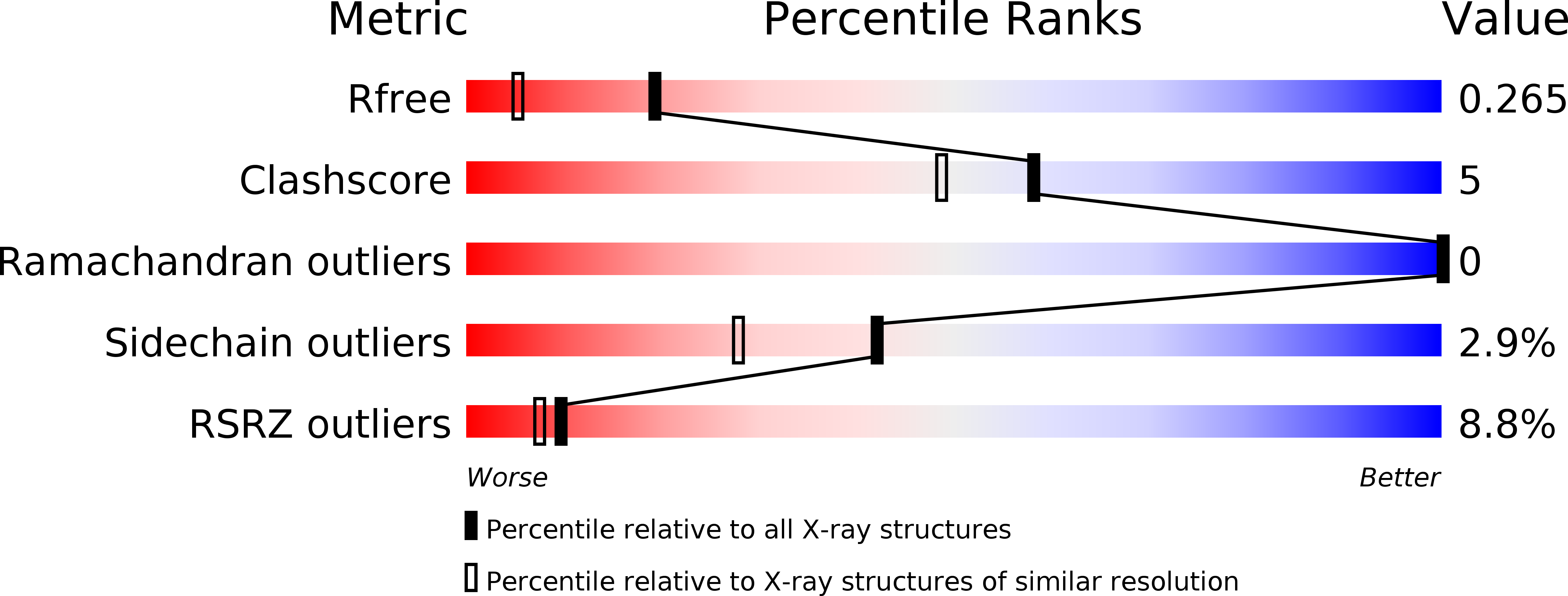
Resolution:
1.82 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 21 21 2