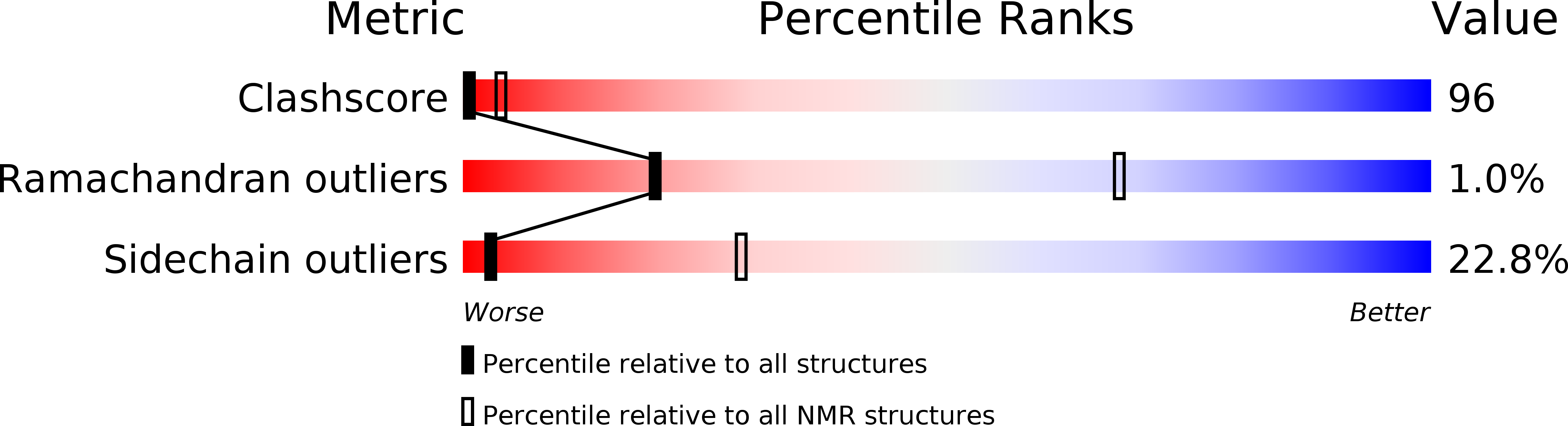
Deposition Date
1997-11-20
Release Date
1998-04-01
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1ZNM
Keywords:
Title:
A zinc finger with an artificial beta-turn, original sequence taken from the third zinc finger domain of the human transcriptional repressor protein YY1 (YING and YANG 1, a delta transcription factor), nmr, 34 structures
Biological Source:
Source Organism:
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
34
Selection Criteria:
NO NOE VIOLATIONS ABOVE 0.2 ANGSTROM AND 5 DEGREE DIHEDRAL ANGLE