Deposition Date
2005-04-01
Release Date
2006-01-03
Last Version Date
2024-04-03
Entry Detail
PDB ID:
1Z9A
Keywords:
Title:
Crystal Structure Of The Asn-309 To Asp Mutant Of Candida Tenuis Xylose Reductase (Akr2B5) Bound To Nad+
Biological Source:
Source Organism:
Candida tenuis (Taxon ID: 45596)
Host Organism:
Method Details:
Experimental Method:
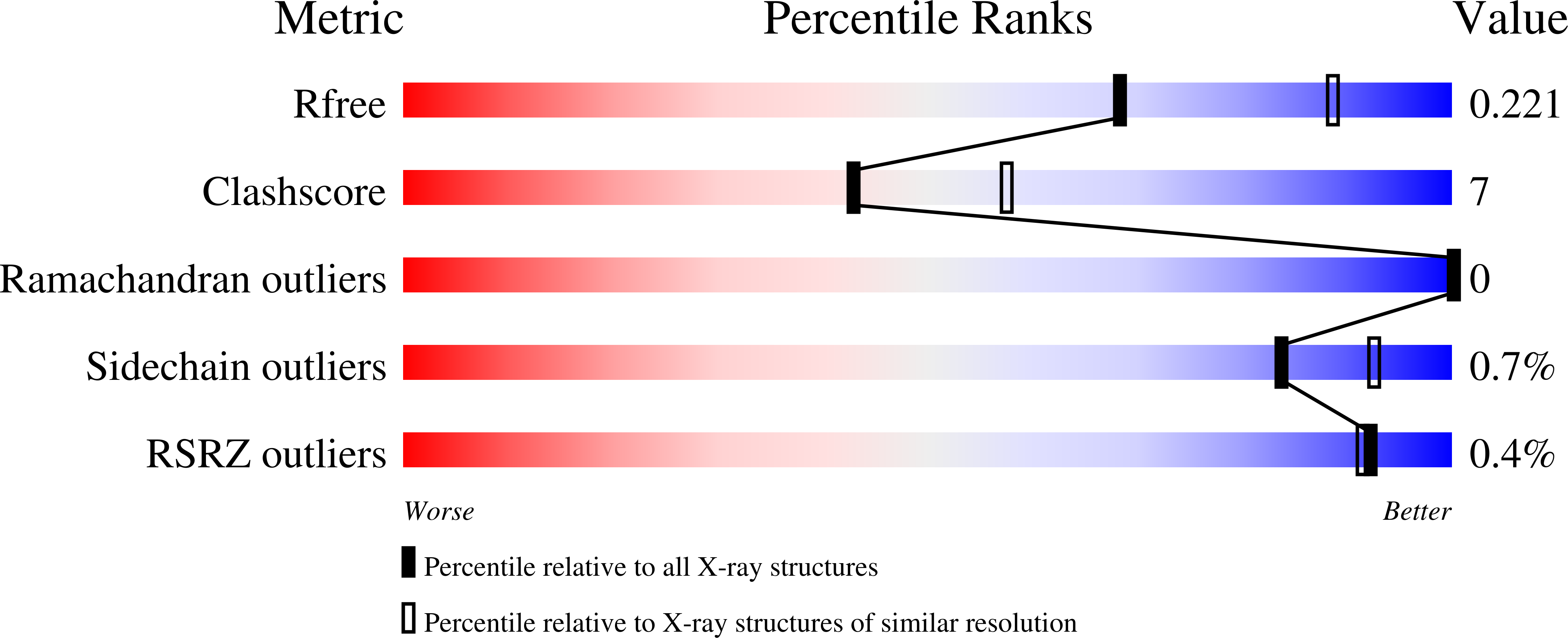
Resolution:
2.40 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1