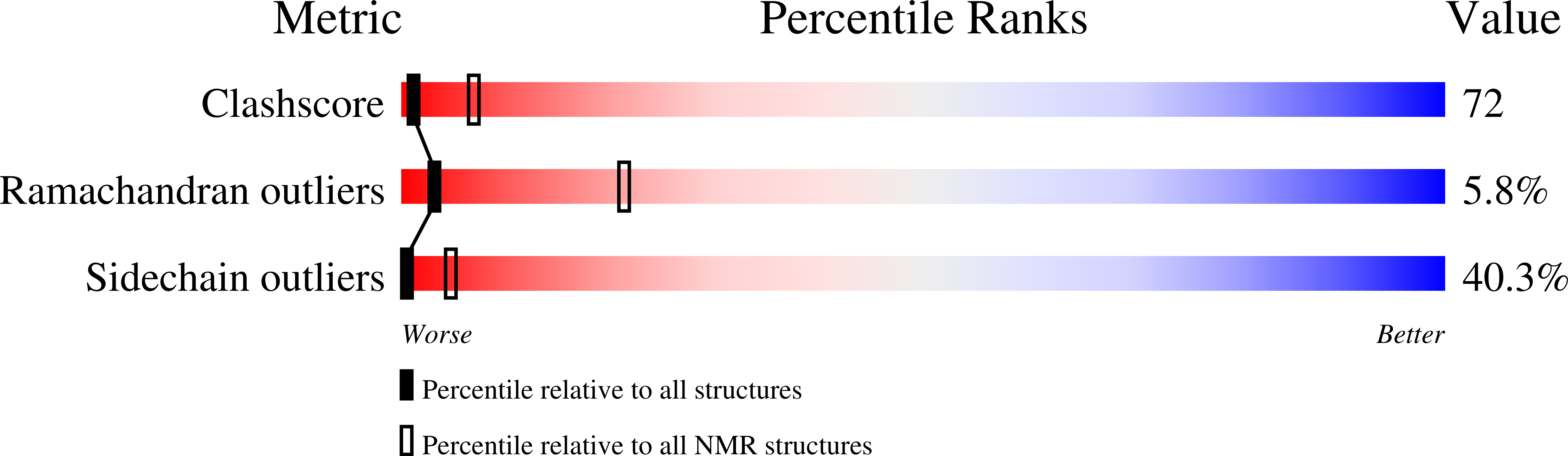
Deposition Date
2005-03-31
Release Date
2005-10-04
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1Z8S
Keywords:
Title:
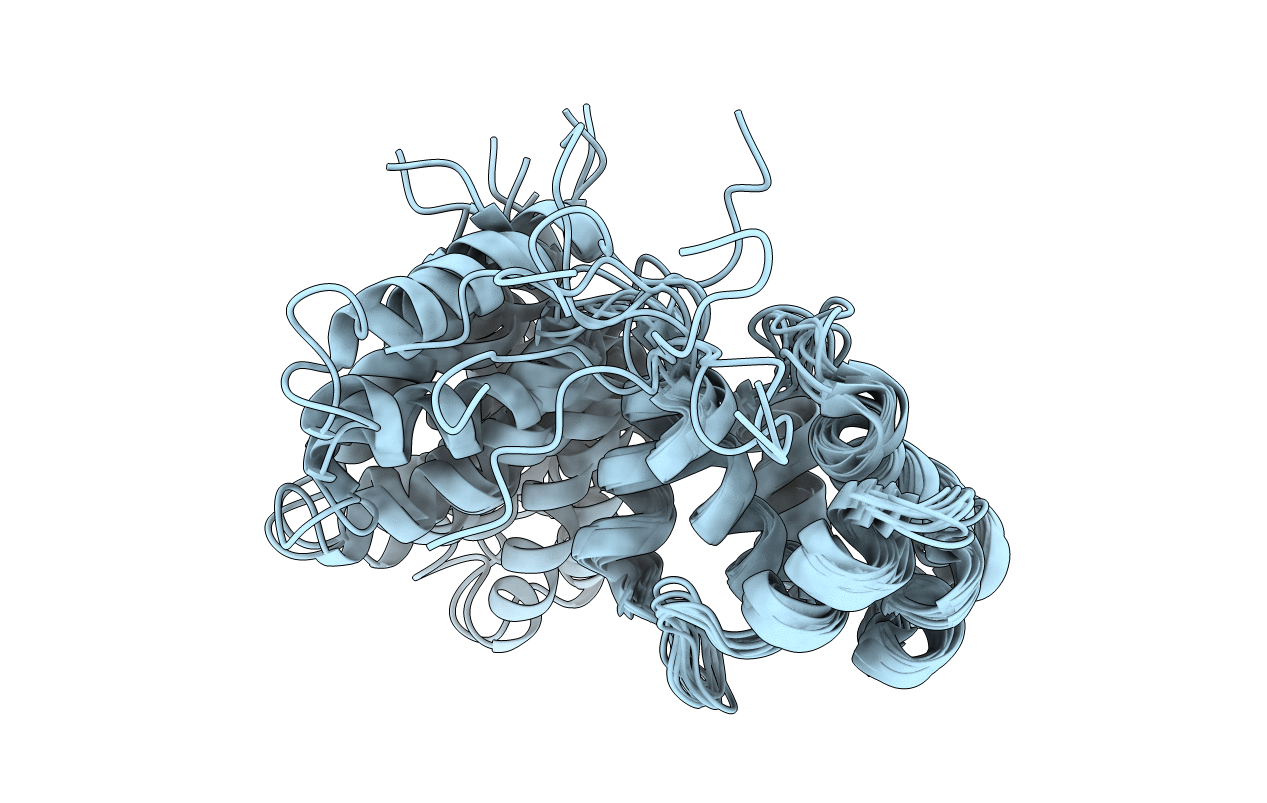
DnaB binding domain of DnaG (P16) from Bacillus stearothermophilus (residues 452-597)
Biological Source:
Source Organism:
Geobacillus stearothermophilus (Taxon ID: 1422)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy